physio-b.sc-unit-10-The Reproductive system
🧬 Structure of the Female Reproductive System
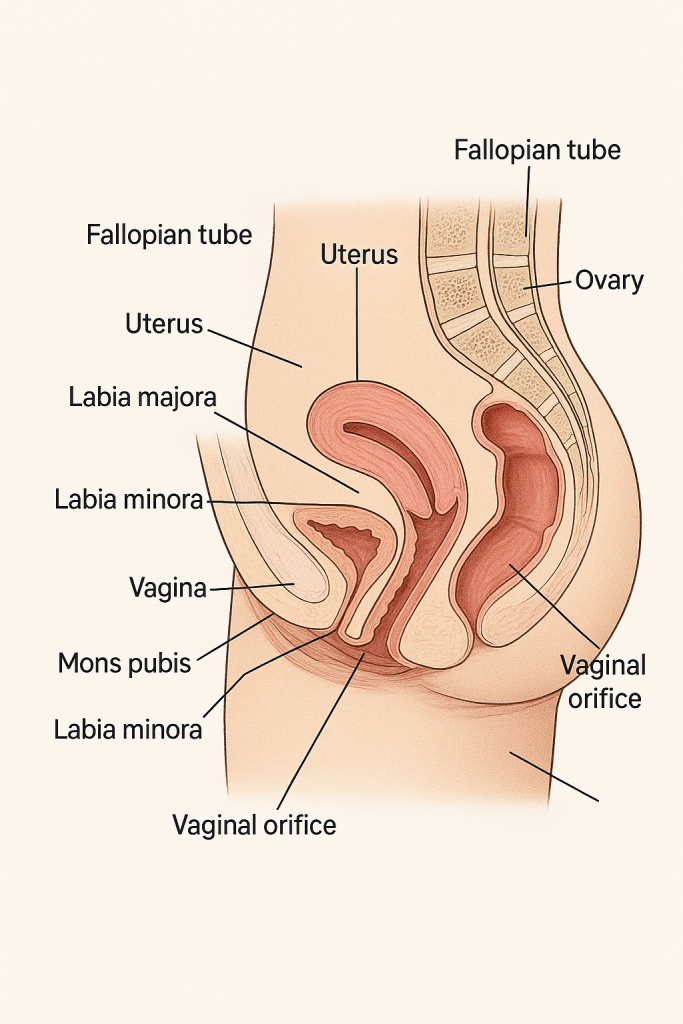
The female reproductive system is a complex network of internal and external organs designed to perform multiple functions: ovum production, fertilization, support of fetal development, and birth. It also plays a crucial role in hormonal regulation and menstruation.
🔸 1. External Genital Organs (Vulva)
The external genitalia, collectively called the vulva, include the following structures:
a) Mons Pubis:
- Fatty tissue overlying the pubic bone.
- Covered with pubic hair after puberty.
- Acts as a cushion during sexual activity.
b) Labia Majora:
- Two large, fleshy folds of skin that extend from the mons pubis to the perineum.
- Contain sebaceous and sweat glands.
- Covered with hair on the outer surface and smooth inside.
c) Labia Minora:
- Thinner, hairless folds of skin lying medial to the labia majora.
- Rich in blood vessels and nerve endings.
- Form the prepuce (hood) and frenulum of the clitoris.
d) Clitoris:
- Small, cylindrical erectile organ located at the anterior junction of the labia minora.
- Richly supplied with nerve endings, responsible for sexual arousal.
e) Vestibule:
- Area enclosed by the labia minora.
- Contains openings of:
- Urethra
- Vagina
- Bartholin’s glands (secrete mucus for lubrication)
f) Perineum:
- Area between the vaginal opening and the anus.
- Muscular and fibrous tissue important in childbirth.
🔹 2. Internal Genital Organs
a) Vagina:
- A muscular, elastic canal ~7–10 cm long.
- Extends from the vaginal orifice to the cervix.
- Functions:
- Receives the penis during intercourse
- Acts as a birth canal during delivery
- Outlet for menstrual flow
- Lined with stratified squamous epithelium.
- Maintains an acidic pH (~4.0–5.0) due to Lactobacillus species.
b) Uterus (Womb):
- Pear-shaped, hollow muscular organ located in the pelvis.
- Size: ~7.5 cm long, 5 cm wide.
- Divided into:
- Fundus (top dome-shaped portion)
- Body (corpus) – middle main portion
- Isthmus – narrow area before the cervix
- Cervix – lower cylindrical portion that opens into the vagina
Uterine Wall Layers:
- Endometrium – inner mucosal layer; undergoes cyclic changes during menstruation.
- Myometrium – thick middle layer of smooth muscle; contracts during labor.
- Perimetrium – outer serous layer (part of peritoneum).
c) Fallopian Tubes (Uterine Tubes / Oviducts):
- Paired tubes, ~10–12 cm long, extend from uterus to ovaries.
- Each tube has 4 parts:
- Fimbriae – finger-like projections that catch the ovum
- Infundibulum
- Ampulla – site of fertilization
- Isthmus
- Lined with ciliated columnar epithelium to help move the ovum toward the uterus.
d) Ovaries:
- Paired almond-shaped glands located on either side of the uterus.
- Size: ~3 × 1.5 × 1 cm.
- Functions:
- Produce ova (eggs) – via oogenesis
- Secrete hormones – mainly estrogen and progesterone
- Structure:
- Cortex – contains ovarian follicles at different stages of development.
- Medulla – contains blood vessels, lymphatics, and nerves.
🔸 3. Accessory Glands
a) Bartholin’s Glands:
- Located near the vaginal opening.
- Secrete mucus to lubricate the vulva during sexual arousal.
b) Skene’s Glands (Paraurethral Glands):
- Located near the urethral opening.
- May contribute to lubrication.
🔹 4. Supporting Structures
- Broad ligament: A peritoneal fold that supports the uterus, fallopian tubes, and ovaries.
- Ovarian ligament: Connects ovary to uterus.
- Round ligament: Maintains anteversion of the uterus.
- Uterosacral ligament: Attaches uterus to the sacrum, supporting the uterus from behind.
- Cardinal (Mackenrodt’s) ligament: Primary support to the cervix and upper vagina.
📌 Summary of Functions
- Ovaries: Gamete production, hormone secretion.
- Fallopian Tubes: Fertilization and ovum transport.
- Uterus: Implantation and nourishment of embryo.
- Vagina: Copulation, childbirth, menstruation outlet.
- External genitalia: Protection and sexual arousal.
🩸 Menstrual Cycle: Definition and Overview
The menstrual cycle is a regular cyclical physiological process in females, occurring approximately every 28 days, that prepares the body for possible pregnancy. It involves hormonal, ovarian, and uterine changes coordinated by the hypothalamic-pituitary-ovarian axis.
- Occurs: From menarche (first menstruation, ~12 years) to menopause (~45–50 years)
- Cycle length: Average is 28 days, but may range from 21–35 days
- Main organs involved: Hypothalamus, pituitary gland, ovaries, and uterus
🧬 Physiology and Phases of the Menstrual Cycle
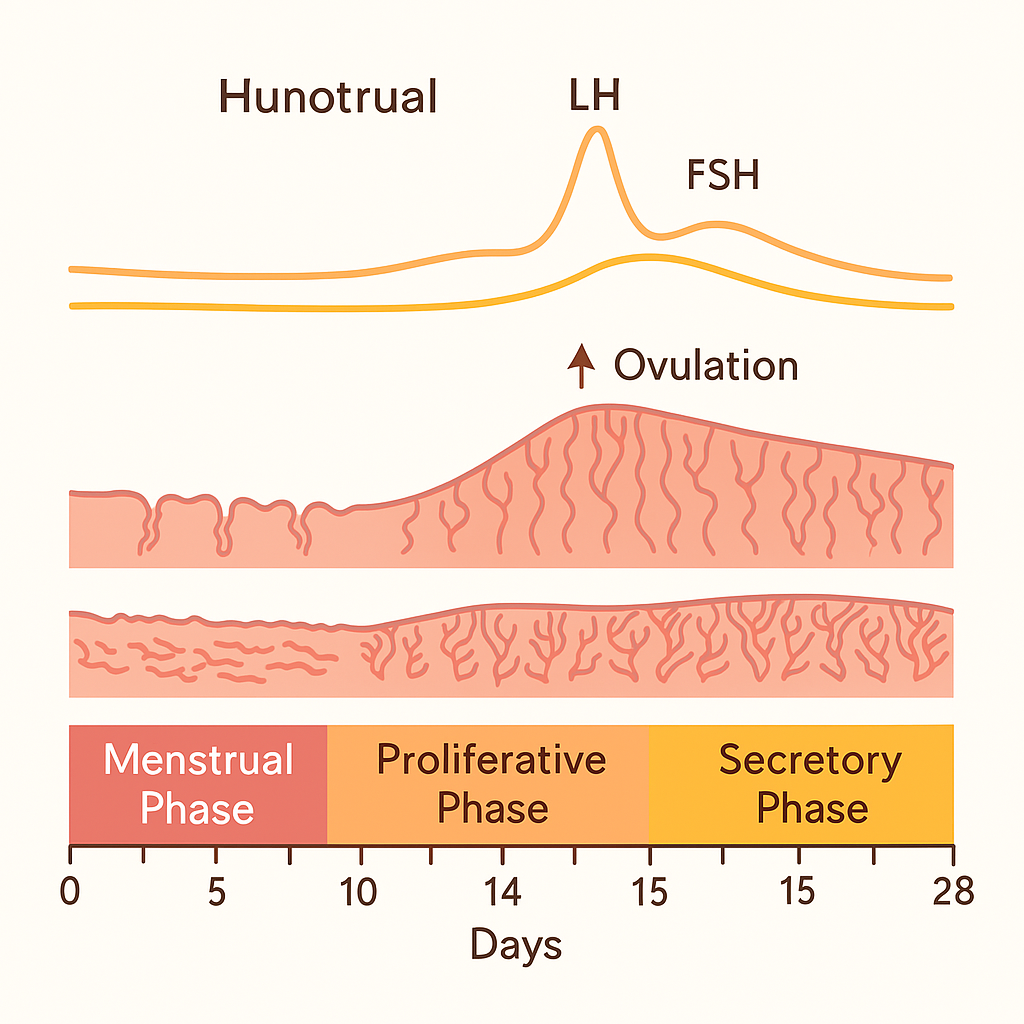
The menstrual cycle is divided into two major cycles:
- Ovarian Cycle (changes in the ovary)
- Uterine (Endometrial) Cycle (changes in the uterus)
These are regulated by the hypothalamic-pituitary-gonadal (HPG) axis.
🔹 1. OVARIAN CYCLE
It consists of three phases:
a) Follicular Phase (Day 1–14)
- Begins on the first day of menstruation.
- The hypothalamus secretes GnRH (Gonadotropin-Releasing Hormone).
- GnRH stimulates the anterior pituitary to release:
- FSH (Follicle Stimulating Hormone): Stimulates growth of primary ovarian follicles.
- LH (Luteinizing Hormone): Supports follicle maturation.
- Developing follicles secrete estrogen, which:
- Stimulates endometrial proliferation
- Inhibits FSH secretion (negative feedback)
- One dominant follicle (Graafian follicle) is selected by ~Day 7.
b) Ovulation (~Day 14)
- A sudden LH surge (triggered by peak estrogen) causes the mature follicle to rupture and release an ovum (egg).
- The egg is picked up by fimbriae of the fallopian tube.
- Ovulation is the most fertile phase of the cycle.
c) Luteal Phase (Day 15–28)
- The ruptured follicle transforms into the corpus luteum under LH influence.
- The corpus luteum secretes progesterone (mainly) and estrogen.
- Progesterone:
- Prepares endometrium for implantation
- Inhibits further LH and FSH (negative feedback)
- If fertilization doesn’t occur, the corpus luteum degenerates after ~14 days into corpus albicans.
- Hormone levels fall, leading to menstruation.
🔸 2. UTERINE (ENDOMETRIAL) CYCLE
This describes the cyclic changes in the endometrial lining in response to ovarian hormones.
a) Menstrual Phase (Day 1–5)
- Occurs due to drop in estrogen and progesterone.
- The functional layer of endometrium is shed, leading to menstrual bleeding (~30–50 mL).
- Accompanied by mild uterine contractions (prostaglandin-mediated).
b) Proliferative Phase (Day 6–14)
- Under estrogen influence from developing follicles:
- The endometrium regenerates and thickens.
- Glands and spiral arteries begin to form.
- Ends with ovulation.
c) Secretory Phase (Day 15–28)
- After ovulation, progesterone from the corpus luteum causes:
- Endometrial glands to secrete nutrients.
- Increased vascularization and preparation for embryo implantation.
- If no implantation, hormone levels drop, causing endometrial breakdown and next menstruation.
🧠 Hormonal Control Summary
| Hormone | Function |
|---|---|
| GnRH | Stimulates FSH & LH release |
| FSH | Stimulates follicular growth |
| LH | Triggers ovulation and supports corpus luteum |
| Estrogen | Builds endometrial lining; triggers LH surge |
| Progesterone | Maintains endometrium; suppresses FSH/LH |
📌 Key Points
- The menstrual cycle is divided into ovarian and uterine components.
- Hormonal interplay between the hypothalamus, pituitary, and ovaries is crucial.
- Ovulation occurs mid-cycle and is the fertile window.
- The cycle resets if fertilization and implantation do not occur.
🧬 Ovary: Structure, Functions, and Hormonal Role
The ovaries are paired female gonads located in the pelvic cavity, attached to either side of the uterus. They are essential for both reproductive and endocrine functions.
🧠 I. Main Functions of the Ovary
1. Gamete Production (Oogenesis)
- The ovaries are responsible for the production of female gametes – ova (eggs).
- This process begins before birth, where primordial germ cells differentiate into oogonia, which later develop into primary oocytes.
- Each menstrual cycle, under hormonal influence, one oocyte matures and is released during ovulation.
- Oogenesis involves:
- Maturation of oocytes in follicles
- Ovulation of a mature ovum (typically one per cycle)
- If not fertilized, the ovum degenerates
2. Hormone Secretion (Endocrine Function)
Ovaries are endocrine organs, producing several steroid hormones and regulatory peptides that control the menstrual cycle and support pregnancy.
🧪 II. Hormones Secreted by the Ovary and Their Functions
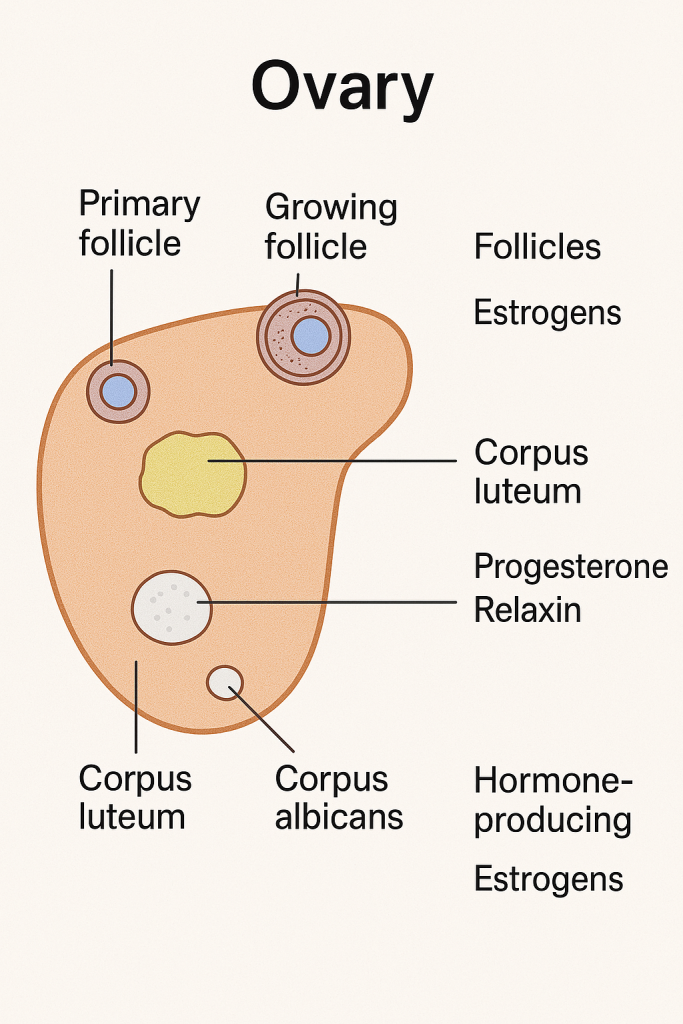
🔹 A. Estrogens
Primary Types:
- Estradiol (E2) – most potent and abundant in reproductive years
- Estrone (E1) – dominant after menopause
- Estriol (E3) – mainly during pregnancy
Secreted By:
- Growing follicles (mainly granulosa cells)
- Corpus luteum
- Placenta (during pregnancy)
Functions:
- Stimulates growth and development of female secondary sexual characteristics (breast development, fat distribution, body hair)
- Promotes endometrial proliferation during the follicular phase
- Enhances growth of uterine muscles
- Maintains the health of the vaginal epithelium
- Stimulates cervical mucus thinning (helps sperm penetration)
- Provides positive feedback to trigger the LH surge for ovulation
- Has protective effects on bones and cardiovascular system
🔸 B. Progesterone
Secreted By:
- Corpus luteum (after ovulation)
- Placenta (during pregnancy)
Functions:
- Prepares the endometrium for implantation (secretory phase)
- Maintains pregnancy (inhibits uterine contractions)
- Stimulates glandular secretion in the uterus
- Causes thickening of cervical mucus (forms a mucus plug)
- Inhibits FSH and LH to prevent multiple ovulations in one cycle
- Contributes to basal body temperature rise post-ovulation
🔹 C. Inhibin
Secreted By:
- Granulosa cells of ovarian follicles
Functions:
- Inhibits FSH secretion from the anterior pituitary (negative feedback)
- Helps regulate follicular recruitment and dominance
🔸 D. Activin
Secreted By:
- Granulosa cells (also by other tissues)
Functions:
- Stimulates FSH secretion
- Promotes follicle development
- Enhances estrogen production
🔹 E. Relaxin
Secreted By:
- Corpus luteum
- Placenta (during pregnancy)
Functions:
- Relaxes the uterine musculature
- Softens the pubic symphysis and cervix during late pregnancy
- Facilitates childbirth
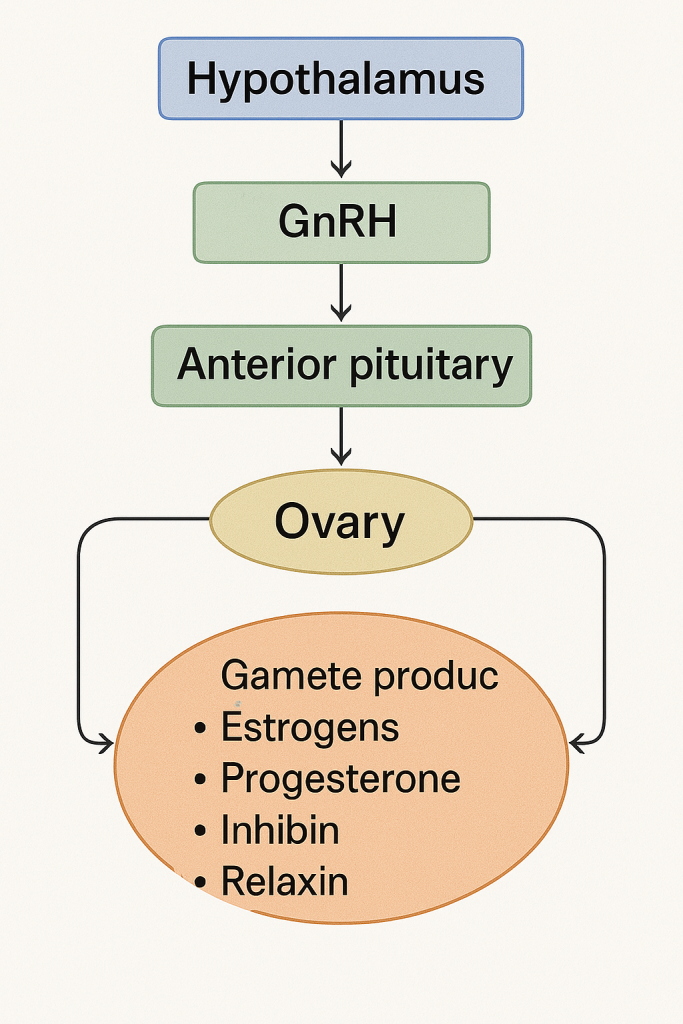
🔄 III. Hormonal Regulation of Ovarian Function
Involves the Hypothalamic-Pituitary-Ovarian Axis:
- Hypothalamus secretes GnRH (Gonadotropin-Releasing Hormone)
- GnRH stimulates the anterior pituitary to release:
- FSH (Follicle Stimulating Hormone) – promotes follicle growth
- LH (Luteinizing Hormone) – triggers ovulation and corpus luteum formation
- Ovary responds by producing estrogen, progesterone, inhibin, relaxin, etc.
- These hormones exert feedback regulation on the hypothalamus and pituitary.
🧠
The ovary plays a dual role:
- Reproductive – by maturing and releasing ova
- Endocrine – by secreting hormones essential for regulating the menstrual cycle, fertility, pregnancy, and female secondary sexual characteristics
Understanding ovarian physiology is essential for diagnosing and managing conditions like infertility, polycystic ovarian syndrome (PCOS), menstrual disorders, and menopause.
🧬 Oogenesis – Definition
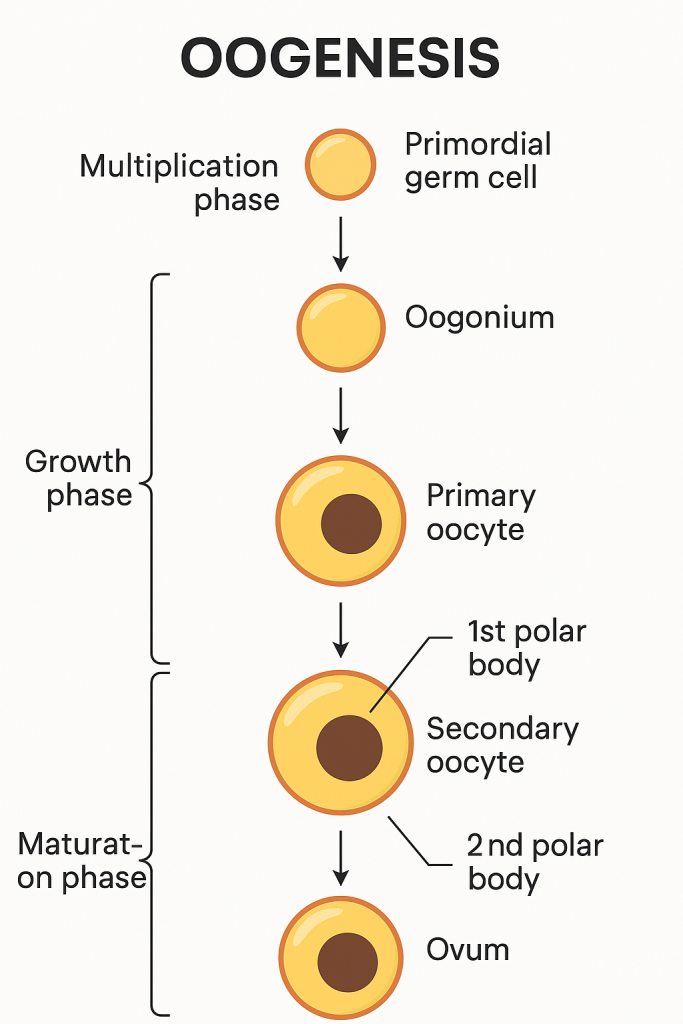
Oogenesis is the process of formation, growth, and maturation of the female gametes (ova or egg cells) in the ovaries. It begins before birth, continues throughout a woman’s reproductive years, and ends at menopause.
🧫 Stages of Oogenesis
Oogenesis occurs in three main stages:
🔹 1. Multiplication Phase (Fetal Life)
- Begins during intrauterine life.
- Primordial germ cells (oogonia) undergo mitosis to multiply.
- By the 5th month of gestation, millions of oogonia are formed in each ovary.
- Most degenerate by atresia; a few survive and differentiate into primary oocytes.
- Each primary oocyte becomes surrounded by follicular cells and forms a primordial follicle.
📌 All primary oocytes are formed before birth and are arrested in prophase I of meiosis.
🔸 2. Growth Phase (Childhood to Puberty)
- During childhood, oocytes remain dormant in prophase I.
- At puberty, under the influence of FSH, some primary oocytes are stimulated to grow.
- The primary oocyte enlarges, accumulates nutrients and cytoplasm.
- The surrounding follicular cells proliferate, forming secondary and tertiary follicles.
🔹 3. Maturation Phase (After Puberty – Menstrual Cycles)
This phase involves completion of meiosis in selected follicles during each menstrual cycle.
a) First Meiotic Division:
- The primary oocyte completes meiosis I just before ovulation.
- Produces:
- One large secondary oocyte
- One small first polar body (non-functional)
b) Second Meiotic Division:
- The secondary oocyte begins meiosis II but is arrested at metaphase II.
- This secondary oocyte is ovulated.
- If fertilization occurs, meiosis II is completed to form:
- One mature ovum
- One second polar body
❗ If no fertilization, the secondary oocyte degenerates without completing meiosis II.
🧪 Summary of Cell Types Involved
| Stage | Cell Type | Ploidy | Status |
|---|---|---|---|
| Primordial Germ Cell → Oogonium | Diploid (2n) | Mitosis | |
| Oogonium → Primary Oocyte | Diploid (2n) | Arrested in Prophase I | |
| Primary Oocyte → Secondary Oocyte + 1st Polar Body | Haploid (n) | Meiosis I complete | |
| Secondary Oocyte → Ovum + 2nd Polar Body (only if fertilized) | Haploid (n) | Meiosis II complete |
🔁 Comparison: Spermatogenesis vs. Oogenesis
- In spermatogenesis, all four meiotic products become functional sperm.
- In oogenesis, only one functional ovum is produced; the other 2–3 cells become polar bodies and degenerate.
🧠 Key Points to Remember
- Oogenesis starts before birth and completes only if fertilization occurs.
- One ovum is produced from each primary oocyte.
- Controlled by FSH and LH under the hypothalamic-pituitary-ovarian axis.
- Aging affects oocyte quality and quantity due to long arrest in meiosis.
🧬 Fertilization – Definition
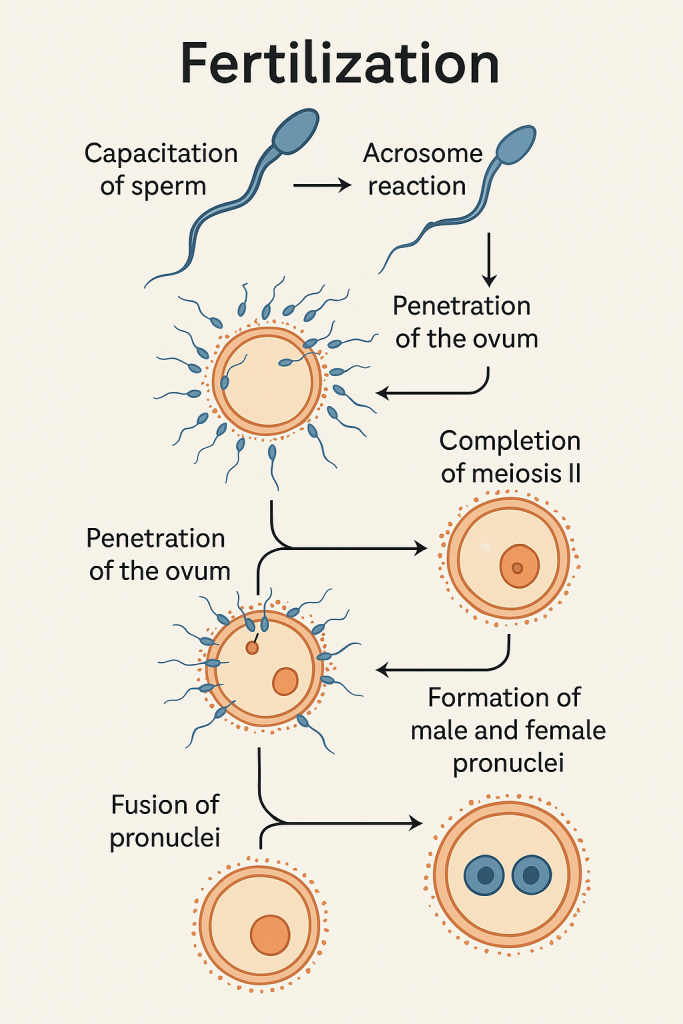
Fertilization is the fusion of a male gamete (sperm) and a female gamete (ovum) to form a single-cell zygote, marking the beginning of human development. It restores the diploid number (46 chromosomes) and initiates the embryonic process.
📍 Site of Fertilization
- Occurs in the ampulla of the fallopian tube, the widest and most lateral portion of the uterine tube.
- The ovum remains viable for 12–24 hours after ovulation.
- Sperm remain viable for up to 3–5 days in the female reproductive tract.
⏱️ Timing of Fertilization
- Usually occurs within 12 to 24 hours after ovulation.
- Most fertile period: 24 hours before and after ovulation.
🔄 Phases of Fertilization
Fertilization occurs in several well-coordinated steps:
🔹 1. Capacitation of Sperm
- Occurs in the female reproductive tract, especially in the uterus or fallopian tube.
- Biochemical changes in the sperm membrane:
- Removal of glycoprotein coat and seminal plasma proteins.
- Increases sperm motility and prepares the sperm for acrosome reaction.
🔸 2. Acrosome Reaction
- Upon contact with the zona pellucida (glycoprotein layer around the ovum), the sperm undergoes:
- Release of enzymes like hyaluronidase and acrosin from the acrosome.
- These enzymes digest a path through the zona pellucida.
🔹 3. Penetration of the Ovum
- The sperm passes through:
- Corona radiata (layer of follicular cells)
- Zona pellucida
- Perivitelline space
- Oocyte membrane (oolemma)
- Only one sperm typically penetrates the ovum.
🔸 4. Cortical Reaction (Block to Polyspermy)
- After the first sperm enters:
- The ovum releases enzymes from cortical granules that modify the zona pellucida.
- Prevents entry of additional sperm (polyspermy block).
🔹 5. Completion of Meiosis II
- Entry of sperm triggers the secondary oocyte to complete meiosis II.
- Produces:
- One mature ovum (nucleus = female pronucleus)
- One second polar body
🔸 6. Formation of Male and Female Pronuclei
- The sperm nucleus enlarges to form the male pronucleus.
- The two pronuclei (male and female) move toward each other.
🔹 7. Fusion of Pronuclei (Syngamy)
- The pronuclei fuse, restoring the diploid number (46 chromosomes).
- This results in the formation of a zygote – a totipotent single cell capable of developing into a full organism.
🧠 Physiological Significance of Fertilization
- Restores diploid number of chromosomes (23 from each parent)
- Determines the sex of the embryo (XX = female, XY = male)
- Initiates cleavage and embryogenesis
- Activates the metabolic machinery of the ovum
- Begins the genetic recombination ensuring variability
⚙️ Factors Influencing Fertilization
- Healthy sperm and ovum
- Proper timing of intercourse (fertile window)
- Patent and functional fallopian tubes
- Favorable cervical mucus and uterine environment
- Absence of immune or anatomical barriers
🚫 Failure of Fertilization
If fertilization does not occur:
- The ovum degenerates within 24 hours.
- Corpus luteum becomes corpus albicans.
- Progesterone and estrogen drop, leading to menstruation.
🧬 Implantation – Definition
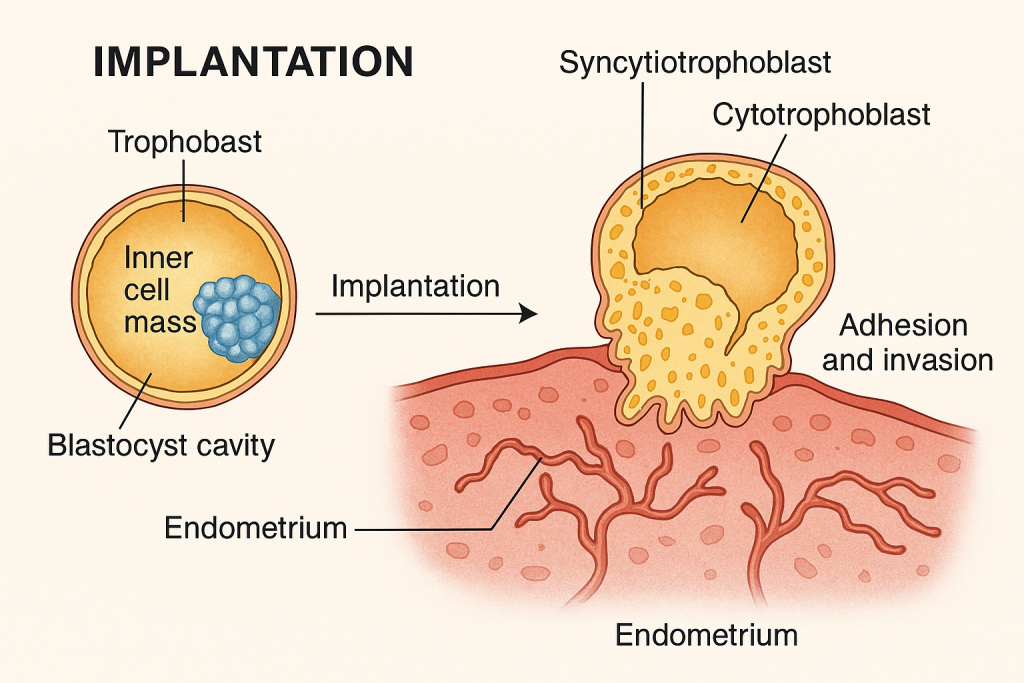
Implantation is the process by which a blastocyst (early-stage embryo) attaches to and invades the endometrial lining of the uterus to establish a maternal-fetal connection. It marks the beginning of pregnancy and is essential for embryo survival and development.
⏱️ Timing of Implantation
- Occurs around 6–7 days after fertilization
- Most commonly between Day 20 to 24 of the menstrual cycle (considering ovulation at Day 14)
- Takes 5–6 days to complete
📍 Site of Implantation
- Usually occurs in the posterior superior wall of the uterus, in the functional layer of the endometrium (decidua)
- The endometrium is in the secretory phase, rich in glands and blood supply
- Ectopic implantation may occur in the fallopian tube, cervix, ovary, or peritoneal cavity (pathological)
🔄 Stages of Implantation
🔹 1. Apposition
- Initial loose contact between the blastocyst and endometrial epithelium
- The blastocyst orients itself, with the inner cell mass facing the endometrium
🔸 2. Adhesion
- Firm attachment of the trophoblast (outer layer of blastocyst) to the endometrial epithelial cells
- Mediated by adhesion molecules (integrins, selectins)
🔹 3. Invasion
- Trophoblast differentiates into:
- Cytotrophoblast (inner layer)
- Syncytiotrophoblast (outer, multinucleated layer)
- Syncytiotrophoblast cells invade the endometrium by:
- Secreting proteolytic enzymes that digest maternal tissue
- Breaking through the epithelium and embedding the blastocyst into the stroma
- Endometrial blood vessels are eroded, and maternal blood enters lacunae forming primitive uteroplacental circulation
🧫 Endometrial Response – Decidual Reaction
- Under progesterone influence, endometrial stromal cells enlarge and become decidual cells
- These cells:
- Nourish the embryo
- Control trophoblast invasion
- Secrete cytokines and growth factors
- The modified endometrium is called the decidua
🧪 Hormonal Regulation of Implantation
- Estrogen: Prepares the endometrium during the proliferative phase
- Progesterone: Maintains the endometrium in secretory phase, essential for implantation
- hCG (Human Chorionic Gonadotropin):
- Secreted by syncytiotrophoblast after implantation
- Maintains the corpus luteum, which continues producing progesterone until the placenta takes over
🧠 Physiological Importance of Implantation
- Establishes nutritional connection between mother and embryo
- Initiates the development of the placenta
- Protects the embryo from maternal immune rejection
- Triggers the secretion of hCG, confirming pregnancy
🚨 Clinical Correlations
- Ectopic Pregnancy: Implantation outside uterus (e.g., in fallopian tube) – life-threatening if ruptured
- Implantation Failure: One of the main causes of infertility and recurrent miscarriage
- Placenta previa: Abnormal implantation near or over the cervical os
- Assisted Reproduction: Implantation is a critical step in IVF (in vitro fertilization)
✅ Key Points
- Implantation starts ~6–7 days post-fertilization in the secretory endometrium
- Requires synchronized embryo and endometrial readiness
- Involves apposition, adhesion, and invasion
- Controlled by progesterone, estrogen, and embryonic signals (hCG)
🧬 Functions of the Breast (Mammary Gland)
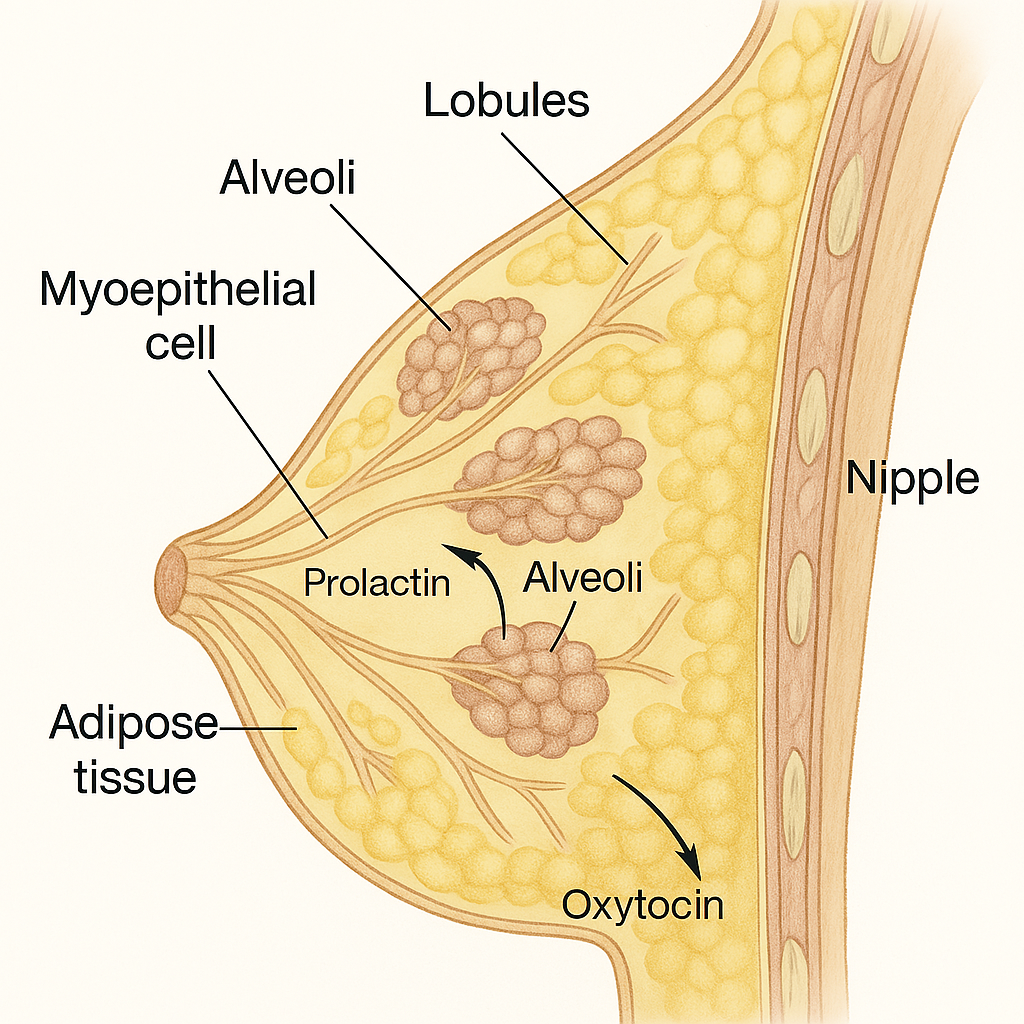
The breasts are paired, modified sweat glands located in the anterior chest wall. In females, they play a key physiological and social role, especially in lactation and reproduction. Structurally, they consist of glandular tissue, ducts, adipose tissue, and supporting connective tissue.
🧠 I. Primary Functions of the Breast
🔹 1. Lactation (Milk Production and Secretion)
This is the main physiological function of the breast.
a) Milk Production:
- Begins after childbirth (lactogenesis), under hormonal control.
- Alveolar cells of the lobules secrete milk.
- Regulated by:
- Prolactin (from anterior pituitary): stimulates milk synthesis.
- Estrogen and progesterone (from the placenta): prepare the breast during pregnancy.
- After delivery, the drop in estrogen/progesterone triggers milk secretion.
b) Milk Ejection (Let-Down Reflex):
- Milk is transported from alveoli to the nipple through lactiferous ducts.
- Controlled by:
- Oxytocin (from posterior pituitary): causes myoepithelial cell contraction, pushing milk out.
- Triggered by suckling stimulus or emotional cues (crying baby).
🔹 2. Nutrition of the Newborn
- Breast milk provides:
- Complete nutrition: proteins (casein, whey), carbohydrates (lactose), fats, vitamins, minerals.
- Immune protection: IgA, lysozyme, lactoferrin, macrophages.
- Growth factors and enzymes beneficial for GI and brain development.
- Colostrum (first milk): Rich in antibodies and nutrients, secreted in the first 2–3 days after delivery.
🔹 3. Immune Protection
- Breast milk is a natural immune defense system for the neonate:
- Contains secretory IgA: coats the gut and prevents pathogen adherence.
- Contains antimicrobial proteins like lactoferrin, lysozyme, and defensins.
- Promotes gut flora colonization with beneficial bacteria (bifidobacteria).
- Reduces risk of infections: respiratory, GI, urinary, and ear infections.
🧠 II. Secondary Functions of the Breast
🔸 4. Endocrine and Reproductive Role
- During puberty, pregnancy, and lactation, the breast responds to hormones:
- Estrogen: promotes ductal growth and breast enlargement.
- Progesterone: promotes alveolar and lobular development.
- Prolactin and oxytocin: critical in lactation.
- Breast development is an important marker of female sexual maturity (Tanner staging).
🔸 5. Psychological and Emotional Bonding
- Breastfeeding enhances maternal-infant bonding through skin-to-skin contact.
- Release of oxytocin fosters emotional connection and maternal behavior.
- Provides comfort, warmth, and security to the infant.
🔸 6. Sexual Function and Aesthetics
- Breasts are secondary sexual characteristics.
- Play a role in sexual arousal and psychological identity.
- May influence self-esteem, body image, and interpersonal relationships.
🔸 7. Social and Cultural Significance
- Breastfeeding is promoted globally for maternal and child health.
- Cultural perceptions vary — breasts may symbolize nurture, femininity, or sexuality depending on societal norms.
🔍 Key Hormones Involved in Breast Functions
| Hormone | Function |
|---|---|
| Estrogen | Ductal growth, breast development during puberty |
| Progesterone | Alveolar/lobular development during pregnancy |
| Prolactin | Milk synthesis post-delivery |
| Oxytocin | Milk ejection (let-down reflex) |
| hPL (Human placental lactogen) | Supports breast growth during pregnancy |
🧠 Clinical Correlations
- Galactorrhea: Milk production unrelated to childbirth (can indicate hormonal imbalance)
- Mastitis: Breast infection during lactation
- Fibrocystic changes: Hormone-related benign changes in the breast
- Breast cancer: A major health concern; regular screening (e.g., mammography) is vital
- Inverted nipple, poor latch: Can affect breastfeeding success
✅
The breast is not just a structure for lactation but a multifunctional organ involved in nourishment, immune defense, reproduction, emotional bonding, and sexual identity. Its functions are intricately regulated by hormonal, neurological, and psychosocial factors.
🧬 Male Reproductive System – Overview
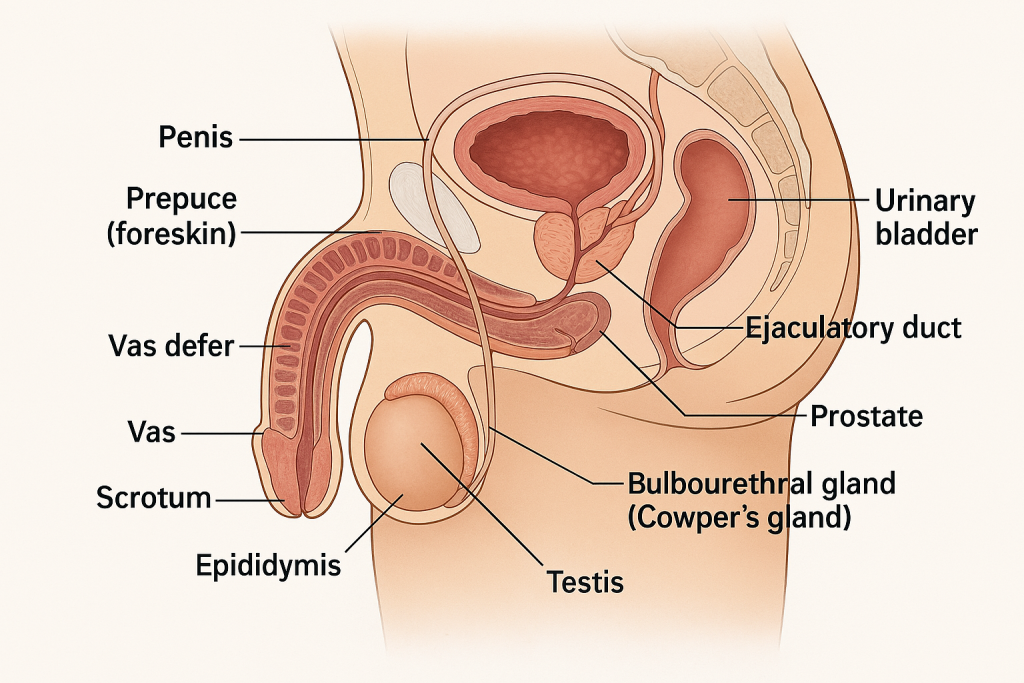
The male reproductive system is responsible for the production, maturation, nourishment, and transport of sperm and the secretion of male sex hormones, primarily testosterone.
It includes external and internal organs and accessory glands, all functioning together to ensure reproductive capability.
🔹 I. Main Components of the Male Reproductive System
🔸 A. External Genital Organs
1. Penis
- Organ of copulation and urine excretion.
- Composed of:
- Three erectile tissues: two corpora cavernosa (dorsal) and one corpus spongiosum (ventral, surrounding the urethra).
- Glans penis: enlarged tip with external urethral meatus.
- Prepuce (foreskin): retractable fold of skin covering the glans.
- Becomes erect during sexual stimulation due to vascular engorgement.
2. Scrotum
- Pouch of skin that houses the testes, epididymis, and part of the spermatic cord.
- Keeps the testes 2–3°C cooler than body temperature—essential for spermatogenesis.
- Contains dartos and cremaster muscles for temperature regulation.
🔸 B. Internal Genital Organs
1. Testes (Testicles)
- Paired oval organs located in the scrotum.
- Function:
- Spermatogenesis: Production of spermatozoa in seminiferous tubules.
- Hormone secretion: Mainly testosterone from Leydig (interstitial) cells.
- Surrounded by tunica albuginea and divided into lobules containing seminiferous tubules.
2. Epididymis
- Long, coiled tube on the posterior side of each testis.
- Site of sperm maturation and storage.
- Sperm gain motility and fertilizing ability here.
3. Vas deferens (Ductus deferens)
- Muscular tube that transports sperm from the epididymis to the ejaculatory duct.
- Part of the spermatic cord; passes through the inguinal canal into the pelvic cavity.
4. Ejaculatory Ducts
- Formed by the union of the vas deferens and seminal vesicle duct.
- Open into the prostatic urethra.
5. Urethra (Male)
- Common pathway for urine and semen.
- Divided into:
- Prostatic urethra
- Membranous urethra
- Penile (spongy) urethra
🔸 C. Accessory Glands
1. Seminal Vesicles
- Paired glands posterior to the bladder.
- Secrete alkaline fluid rich in fructose, prostaglandins, and coagulating enzymes.
- Contributes ~60% of semen volume; nourishes sperm.
2. Prostate Gland
- Single gland surrounding the urethra just below the bladder.
- Produces a milky, slightly acidic fluid containing:
- Citric acid, enzymes (PSA), and antimicrobial proteins.
- Adds ~25–30% of semen volume.
3. Bulbourethral Glands (Cowper’s Glands)
- Pea-sized glands beneath the prostate.
- Secrete mucus-like pre-ejaculatory fluid that lubricates the urethra and neutralizes acidic urine residue.
🧠 II. Functions of the Male Reproductive System
- Sperm Production (Spermatogenesis) in seminiferous tubules
- Hormone Secretion – primarily testosterone, regulating male sexual characteristics
- Sperm Maturation in epididymis
- Semen Production and Ejaculation
- Fertilization Capability – delivering sperm to the female reproductive tract
⚙️ III. Hormonal Control of Male Reproductive Function
Regulated by the hypothalamic-pituitary-gonadal (HPG) axis:
| Hormone | Source | Function |
|---|---|---|
| GnRH | Hypothalamus | Stimulates pituitary to release FSH and LH |
| FSH | Anterior Pituitary | Stimulates Sertoli cells for spermatogenesis |
| LH | Anterior Pituitary | Stimulates Leydig cells to secrete testosterone |
| Testosterone | Testes (Leydig cells) | Promotes sperm formation, secondary sex characteristics |
| Inhibin | Sertoli cells | Inhibits FSH secretion (negative feedback) |
🧪 Semen Composition
- Volume: ~2–5 mL per ejaculation
- pH: 7.2–8.0
- Components:
- Spermatozoa (~10%)
- Seminal fluid from seminal vesicles, prostate, and bulbourethral glands (~90%)
- Fructose, enzymes, zinc, and buffers
🚨 Clinical Correlations
- Infertility: Due to low sperm count, motility, or structural defects
- Prostatitis: Inflammation of the prostate gland
- Testicular torsion: Medical emergency causing loss of blood supply to the testis
- Benign prostatic hyperplasia (BPH): Common in aging males
- Prostate cancer: One of the most common male cancers
✅
The male reproductive system is a complex structure designed to:
- Produce and mature sperm
- Secrete testosterone
- Deliver sperm for fertilization It is controlled by hormonal feedback loops, with each part contributing to the reproductive process.
🧬 Spermatogenesis – Definition
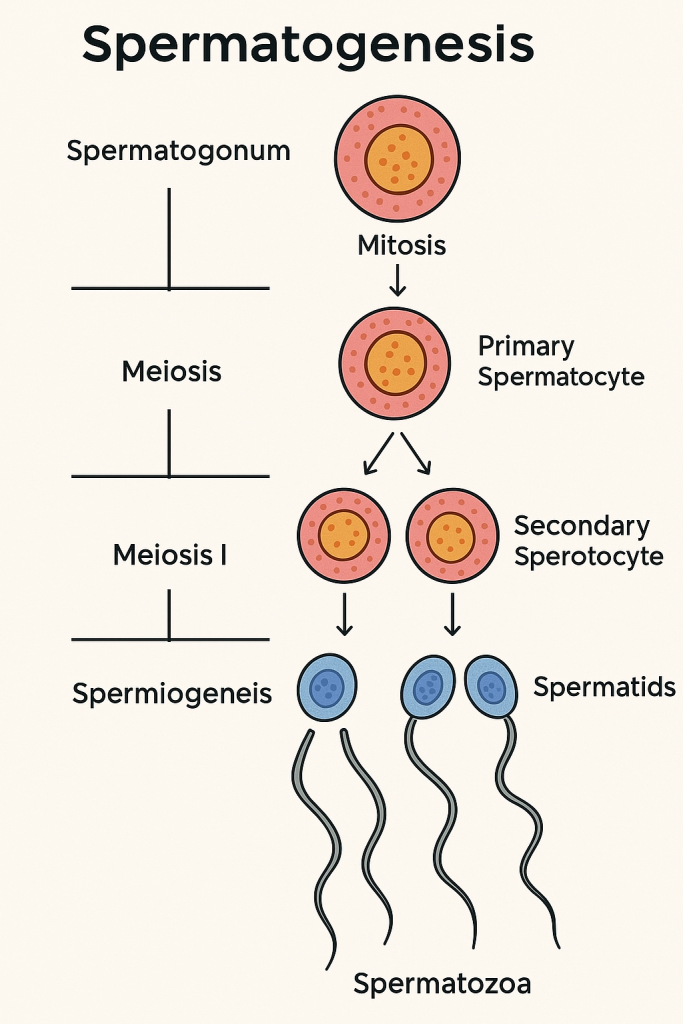
Spermatogenesis is the process by which male primordial germ cells (spermatogonia) develop into mature spermatozoa (sperm cells). This process occurs within the seminiferous tubules of the testes and is vital for male fertility.
It involves a series of mitotic, meiotic, and differentiation phases, supported by the structural and hormonal environment of the testes.
📍 Location of Spermatogenesis
- Takes place in the seminiferous tubules of the testes
- Involves support from:
- Sertoli cells (nourishment, structural support)
- Leydig cells (secretion of testosterone)
⏱️ Timing
- Begins at puberty and continues throughout life.
- Takes about 64–74 days to complete in humans.
- Sperm mature further in the epididymis (~12 days).
🔄 Phases of Spermatogenesis
Spermatogenesis consists of three major phases:
🔹 1. Spermatocytogenesis (Mitotic Phase)
- Begins with spermatogonia (diploid stem cells) located on the basement membrane of seminiferous tubules.
- Spermatogonia Type A:
- Act as stem cells, divide mitotically.
- Some remain as reserve cells, others differentiate.
- Spermatogonia Type B:
- Undergo further mitosis and differentiate into primary spermatocytes.
🔸 2. Meiosis (Reduction Phase)
This phase reduces the chromosome number from diploid (2n) to haploid (n).
a) Primary Spermatocytes (2n)
- Enter Meiosis I to form:
- Two secondary spermatocytes (haploid, n)
b) Secondary Spermatocytes (n)
- Rapidly enter Meiosis II to form:
- Four spermatids (haploid, n)
One primary spermatocyte ultimately gives rise to four spermatids.
🔹 3. Spermiogenesis (Differentiation Phase)
- Spermatids undergo morphological changes to become spermatozoa (mature sperm).
- No cell division occurs; only structural modifications.
Key changes during Spermiogenesis:
- Nucleus condenses – becomes streamlined
- Acrosome formation – develops from Golgi apparatus; contains enzymes for oocyte penetration
- Flagellum (tail) formation – for motility
- Mitochondria align in the midpiece – to supply energy for movement
- Cytoplasm is reduced – residual bodies are phagocytosed by Sertoli cells
The result: fully differentiated non-motile spermatozoa, released into the lumen of the seminiferous tubules (called spermiation).
🧪 Hormonal Regulation of Spermatogenesis
Controlled by the Hypothalamic-Pituitary-Gonadal (HPG) axis:
| Hormone | Source | Function |
|---|---|---|
| GnRH | Hypothalamus | Stimulates FSH and LH secretion |
| FSH | Anterior pituitary | Stimulates Sertoli cells to support spermatogenesis |
| LH | Anterior pituitary | Stimulates Leydig cells to produce testosterone |
| Testosterone | Leydig cells | Essential for meiosis and spermatid maturation |
| Inhibin | Sertoli cells | Provides negative feedback to reduce FSH secretion |
🧠 Sertoli Cells – “Nurse Cells”
- Provide physical and nutritional support to developing sperm
- Form the blood-testis barrier
- Secrete androgen-binding protein (ABP) to maintain high local testosterone
- Release inhibin and activin for feedback control
🔍 Summary of Cell Stages in Spermatogenesis
| Cell Type | Chromosome (Ploidy) | Division Type | Outcome |
|---|---|---|---|
| Spermatogonia (Type A & B) | 2n | Mitosis | Primary spermatocytes |
| Primary spermatocyte | 2n | Meiosis I | 2 Secondary spermatocytes |
| Secondary spermatocyte | n | Meiosis II | 4 Spermatids |
| Spermatid | n | Differentiation (Spermiogenesis) | Spermatozoa |
✅ Key Features
- Continuous process from puberty onward
- Produces millions of sperm daily
- Requires optimal temperature (~2–3°C lower than body temperature)
- Entire process from spermatogonia to motile sperm takes ~70–80 days
🚨 Clinical Relevance
- Oligospermia: Low sperm count
- Azoospermia: Absence of sperm
- Sertoli-cell-only syndrome: No germ cells, causing infertility
- Varicocele, infection, or hormonal imbalance can impair spermatogenesis
🧬 Hormones of the Male Reproductive System – Detailed Overview
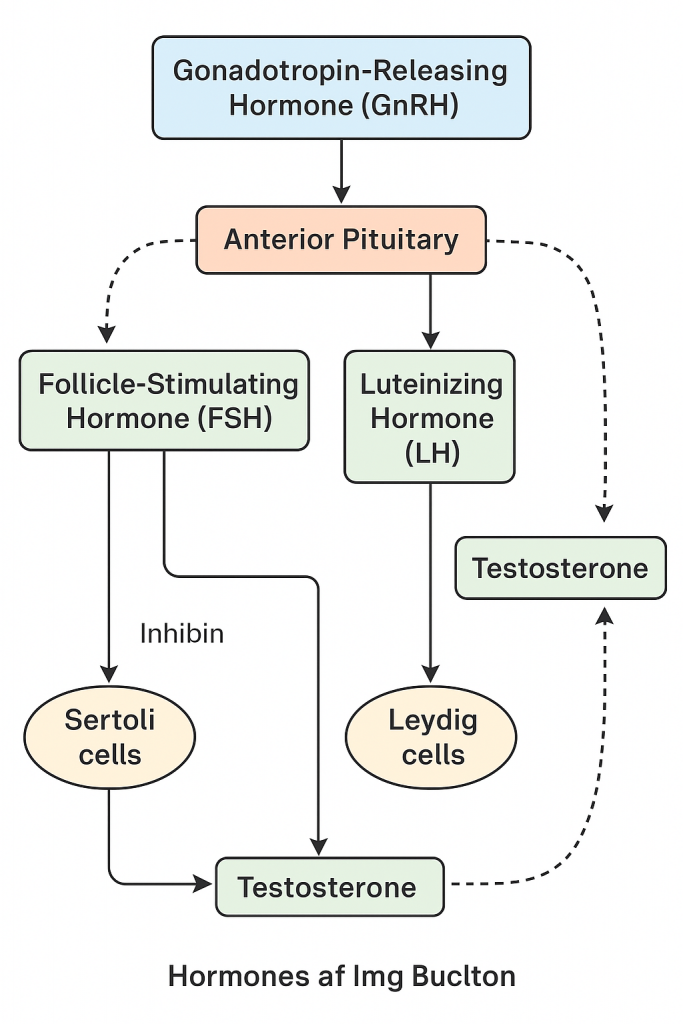
The male reproductive system is regulated by a complex interaction of hormones controlled by the hypothalamic-pituitary-gonadal (HPG) axis. These hormones are responsible for:
- Sexual development during puberty
- Sperm production (spermatogenesis)
- Maintenance of secondary sexual characteristics
- Fertility and libido
🔹 I. Hormones Involved and Their Sources
1. Gonadotropin-Releasing Hormone (GnRH)
- Source: Hypothalamus (Arcuate nucleus)
- Function:
- Stimulates the anterior pituitary to release FSH and LH
- Secreted in a pulsatile manner, critical for normal reproductive function
2. Follicle Stimulating Hormone (FSH)
- Source: Anterior pituitary (adenohypophysis)
- Target: Sertoli cells in the seminiferous tubules
- Functions:
- Stimulates spermatogenesis
- Enhances production of androgen-binding protein (ABP) which helps maintain high testosterone concentration in the testes
- Promotes secretion of inhibin from Sertoli cells (negative feedback to FSH)
- Supports nourishment and maturation of sperm within the seminiferous tubules
3. Luteinizing Hormone (LH)
- Source: Anterior pituitary
- Target: Leydig cells in the testes
- Functions:
- Stimulates Leydig cells to produce and secrete testosterone
- Essential for maintaining intratesticular testosterone levels required for spermatogenesis
4. Testosterone
- Source: Leydig cells of the testes
- Nature: Primary androgen (male sex hormone)
- Functions:
- Promotes development of male reproductive organs (penis, scrotum, seminal vesicles, prostate)
- Induces secondary sexual characteristics: deep voice, facial/body hair, increased muscle mass, male body pattern of fat distribution
- Stimulates libido and sexual behavior
- Supports spermatogenesis in synergy with FSH
- Inhibits GnRH, LH, and FSH via negative feedback
- Promotes erythropoiesis (increases red blood cell count)
5. Inhibin
- Source: Sertoli cells of the testes
- Functions:
- Inhibits FSH secretion from the anterior pituitary (negative feedback)
- Regulates the rate of spermatogenesis
- Marker of Sertoli cell function
6. Activin
- Source: Sertoli cells and other tissues
- Functions:
- Opposite of inhibin: stimulates FSH secretion
- Enhances spermatogenesis by supporting Sertoli cell activity
7. Androgen-Binding Protein (ABP)
- Source: Sertoli cells (in response to FSH)
- Function:
- Binds to testosterone and maintains high local concentration in the seminiferous tubules
- Essential for maturation of sperm cells
8. Estrogens (minor role in males)
- Source: Conversion of testosterone via aromatase enzyme in Sertoli cells, liver, and adipose tissue
- Functions:
- Support sperm maturation
- Regulate fluid reabsorption in the epididymis
- May influence bone metabolism, libido, and brain function
🧠 II. Hormonal Axis: Hypothalamic–Pituitary–Testicular (HPT) Axis
Step-by-Step Mechanism:
- Hypothalamus secretes GnRH → stimulates anterior pituitary.
- Anterior Pituitary releases:
- FSH → stimulates Sertoli cells (sperm support and inhibin release)
- LH → stimulates Leydig cells to produce testosterone
- Testosterone and Inhibin → provide negative feedback to hypothalamus and pituitary to control hormone levels.
📌 Summary Table of Male Reproductive Hormones
| Hormone | Source | Target | Key Function |
|---|---|---|---|
| GnRH | Hypothalamus | Anterior pituitary | Stimulates FSH & LH |
| FSH | Anterior pituitary | Sertoli cells | Supports spermatogenesis |
| LH | Anterior pituitary | Leydig cells | Stimulates testosterone production |
| Testosterone | Leydig cells | Multiple | Secondary sex traits, spermatogenesis |
| Inhibin | Sertoli cells | Anterior pituitary | Inhibits FSH |
| Activin | Sertoli cells | Anterior pituitary | Stimulates FSH |
| ABP | Sertoli cells | Seminiferous tubules | Maintains testosterone levels for sperm development |
✅ Clinical Relevance
- Hypogonadism: Low testosterone levels → delayed puberty, infertility
- Klinefelter syndrome: Testicular failure due to chromosomal abnormality
- Andropause: Age-related decline in testosterone production
- Infertility: Due to hormonal imbalance affecting spermatogenesis