ENGLISH-FON:Unit: 6(Part : 3 )Care of patient with Fluid and Electrolyte imbalance:-UPLOAD
Unit: 6 Therapeutic Nursing care
Part: 3 Care of patient with Fluid and Electrolyte imbalance
Key terms:
define acidosis:
In this acid-base imbalance, the concentration of hydrogen ions (H+) increases and its pH becomes less than 7.35. In this, arterial pH is also low due to decreased concentration of bicarbonate (HCO3-), which is called metabolic acidosis.
In respiratory acidosis, the concentration of PCO2 increases, due to which arterial pH is low.
Define alkalosis:

In this acid base imbalance, the concentration of hydrogen (H+) ions decreases, due to which the pH of the blood increases above 7.45. In this, the arterial pH is high due to the concentration of bicarbonate (Hco3-) and metabolic Alkalosis is observed. In respiratory alkalosis, the concentration of pCO2 decreases, which leads to an increase in arterial pH.
Define Dehydration :

Water/fluid loss from the extracellular space. This occurs when the amount of fluid excreted from the body exceeds the intake, causing the amount of fluid in the body to decrease, resulting in a condition of dehydration and an excessively low level of fluid in the body. Therefore, the condition of dehydration arises in the body.
Define Anaphylactic Reactions:
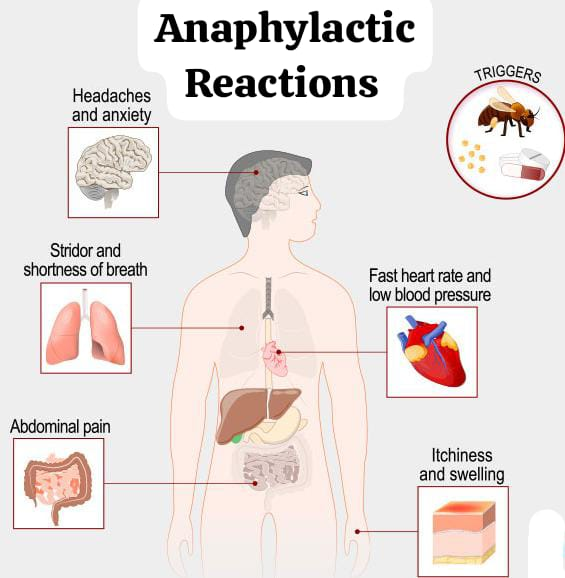
Anaphylactic reactions are life-threatening conditions that cause respiratory and cardiovascular collapse and severe disturbance of the gastrointestinal tract.
Define Blood transfusion:
Blood transfusion is a procedure in which blood and its components are administered into the client’s body.
define osmosis:
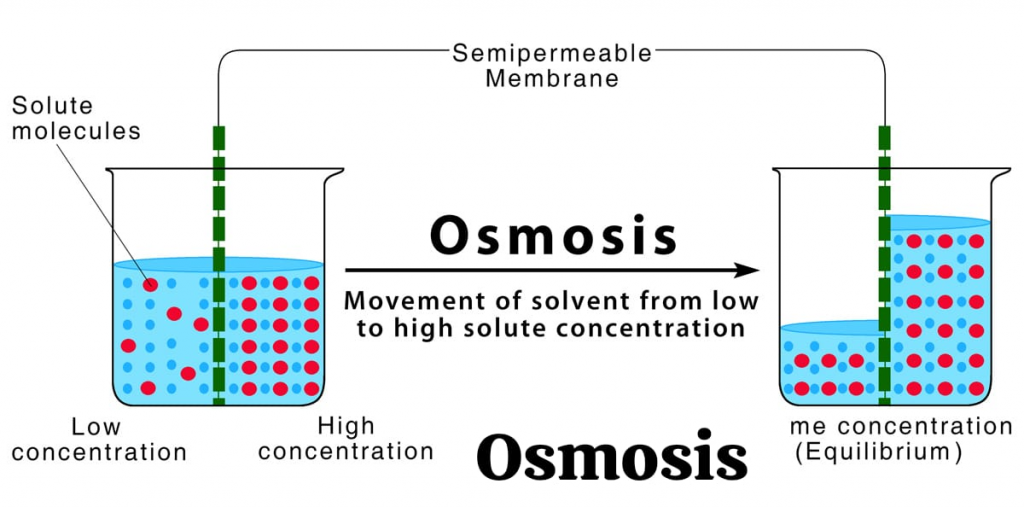
Osmosis is a process in which a solvent moves from a lower concentration to a higher concentration and continues to move until the concentration on both sides of a semipermeable membrane is equal.
define diffusion:
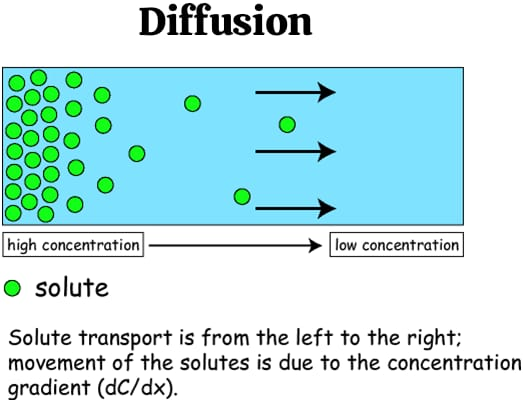
Diffusion is the condition in which molecules of solutes move from a higher solute concentration to a lower solute concentration.
define electrolyte:
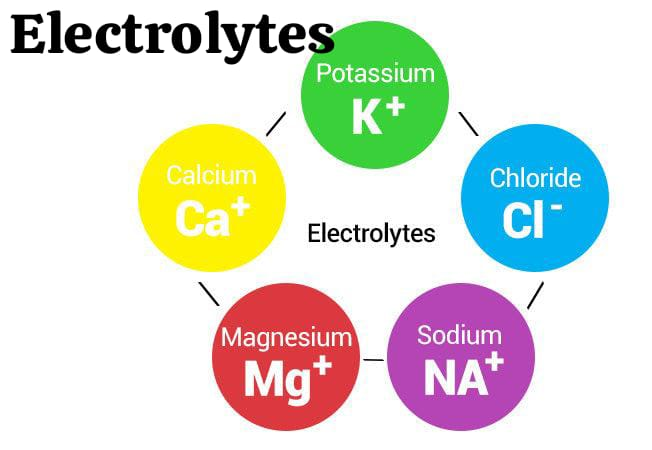
Electrolytes are salts or charges that conduct electricity and are bound to body fluids, such as blood. are formed and their charge is either positive( + ) or negative( – ).
Ex:=Na+,cl-.
define Edema(in Edema):
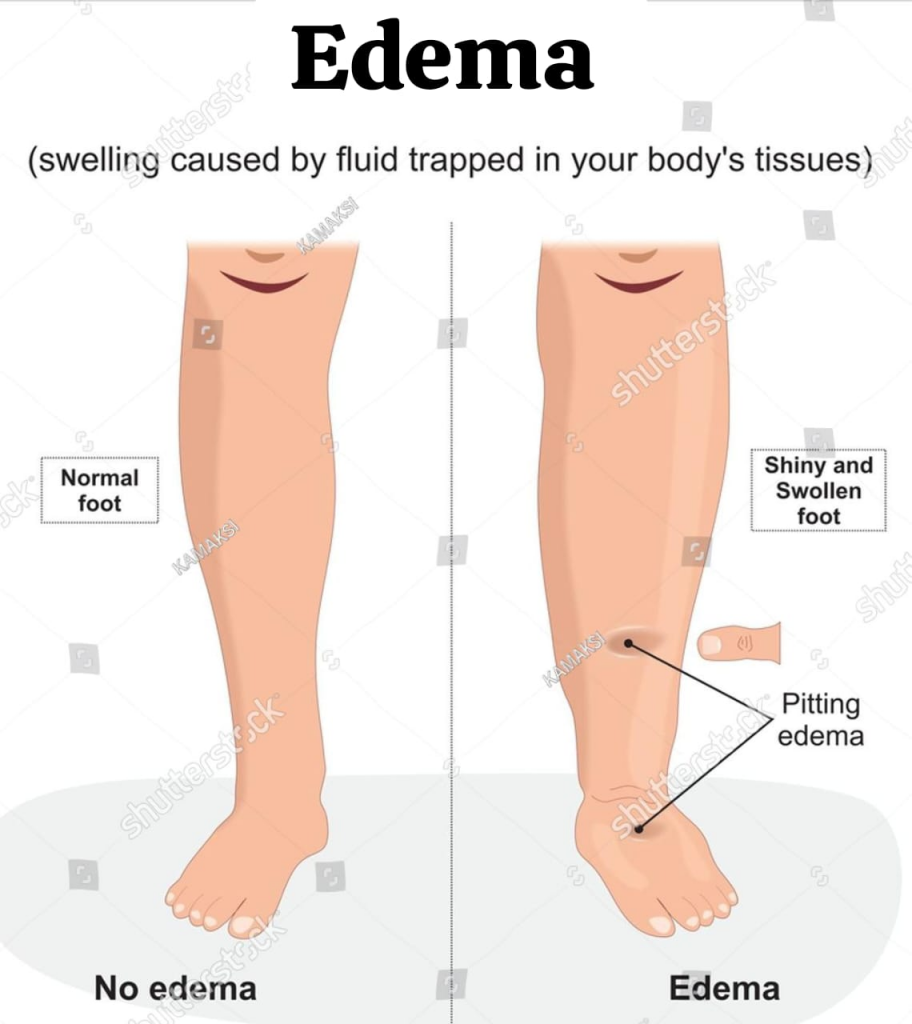
In edema, fluid collects in excess amounts and increases the amount of interstitial fluid.
define intracellular fluid:
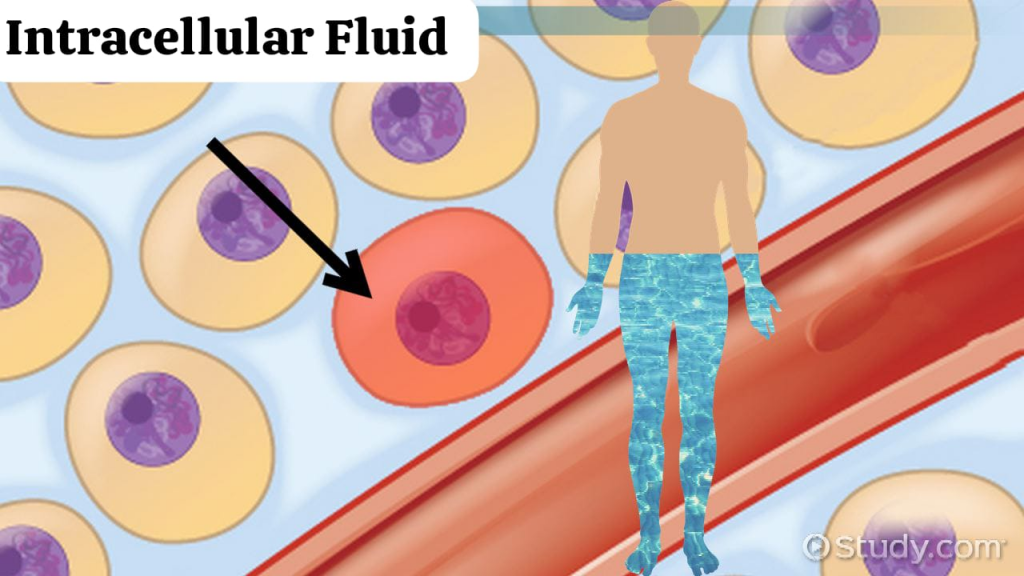
Intracellular fluid is the fluid that is located inside the cell and mainly consists of potassium (k+), magnesium (mg4+) and phosphate (po43-) ions. Intracellular fluid is 70% of the total body water. It is the largest component of the adult body. Makes 40% of the weight.
define extracellular fluid:
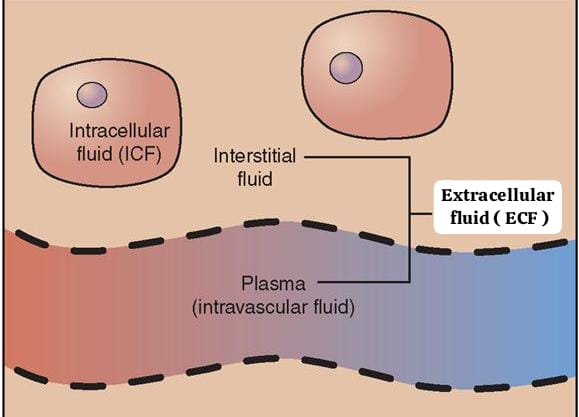
Extracellular fluid is the fluid that is outside the cells. It constitutes 30% of the total body water. It makes up 20% of an adult’s body weight.
Example:= Na+ (sodium ions).
Define filtration:
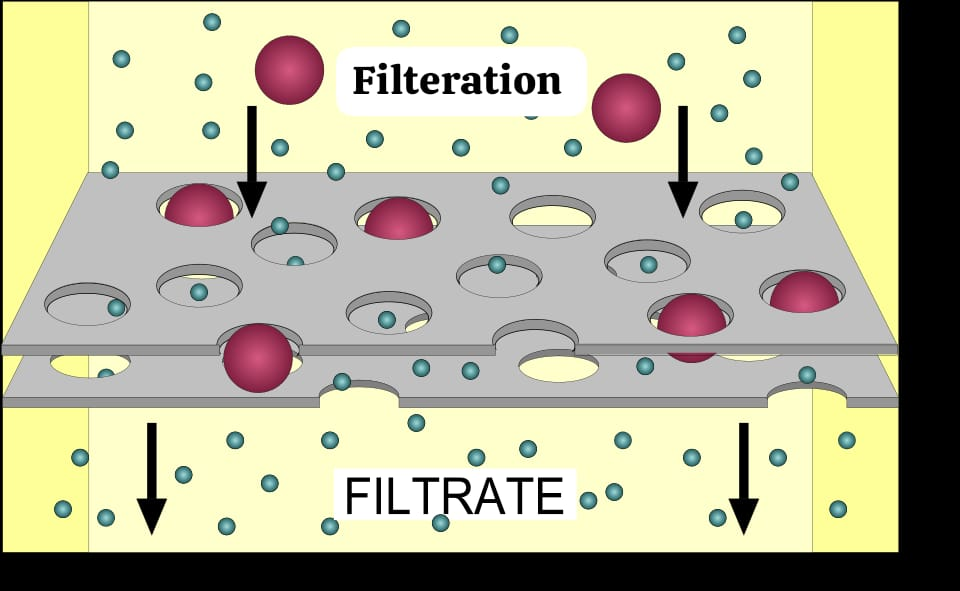
Filtration is the process by which water and dissolved substances move from a high hydrostatic pressure to a low hydrostatic pressure.
Define hypervolemia:
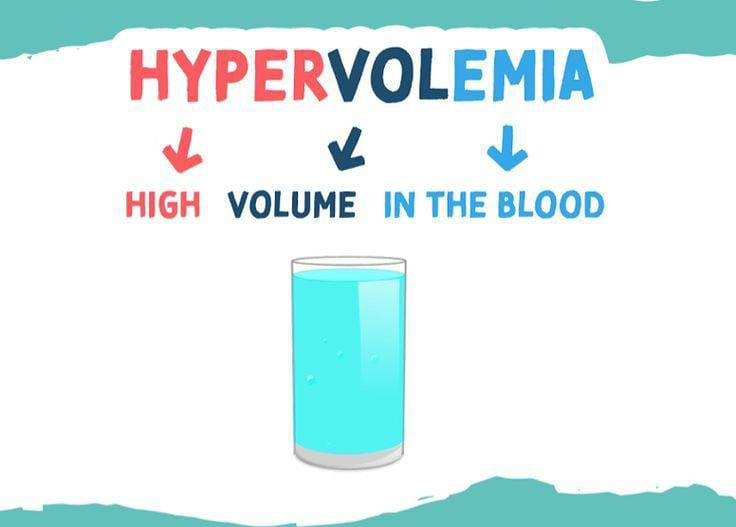
Hypervolemia is a condition in which the amount of fluid in the body increases. Hypervolemia or fluid overload is a type of medical condition.
define hypovolemia (હીપોવોલેમિયા) :

Hypovolemia is a condition in which the body loses more fluid than it takes in.
define acid(असिड):
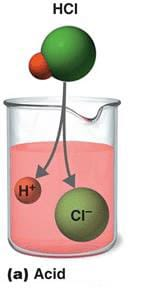
Acid is a compound that forms hydrogen ions in solution.
define base(base):
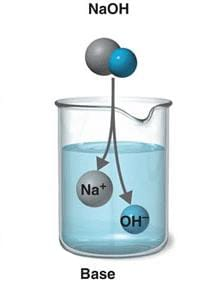
A base is a compound that combines with hydrogen ions in its solution.
define buffer:

A buffer is a substance that prevents major changes in pH (Ph). It keeps a solution neutral by removing and releasing hydrogen ions.
Fluid and Electrolyte Balance:
define electrolyte:
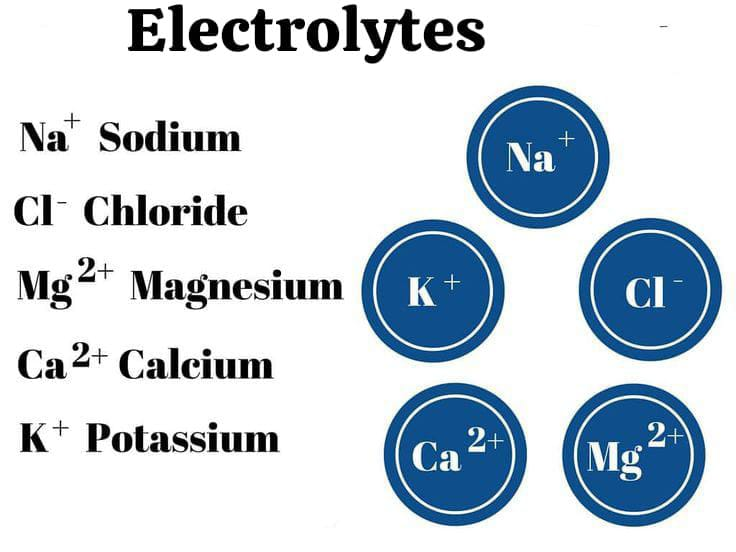
Electrolytes are salts or charges that conduct electricity and are bound to the body’s fluids, blood, and their charge is positive (+) or negative (-).
Ex:=Na+,cl-.
Electrolytes are liquids or solutions of substances that are capable of conducting electricity and are in the form of ions. Dissociates. Electrolytes are salts that conduct electricity and are present in body fluids, tissues, and blood. Cellular function requires a circulating body fluid system that transports nutrients and removes metabolic waste.
Functions of body fluids:
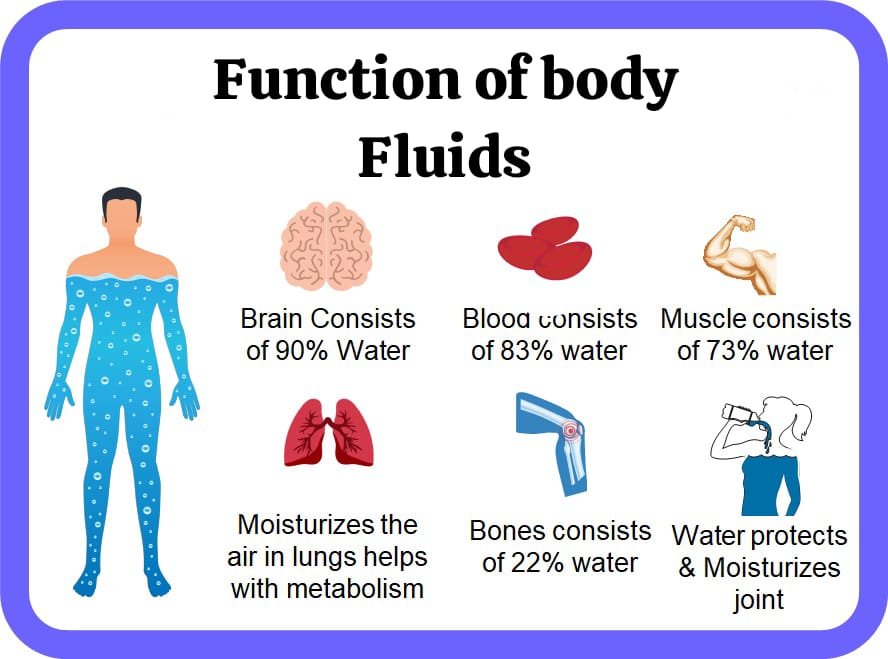
- Works to regulate body temperature.
- Protects vital organs and acts as a cushion. Helps in transporting hormones to activity sites.
- Helps in lubricating joint spaces.
- Maintains hydrostatic pressure in the cardiovascular system.
- Improves intestinal health and prevents constipation.
- Flushes the kidneys of metabolic waste products.
- Works to transport nutrients to cells.
- Helps convert food into energy.
- Helps transport waste products away from cells.
Volume And Distribution of Body Fluids:
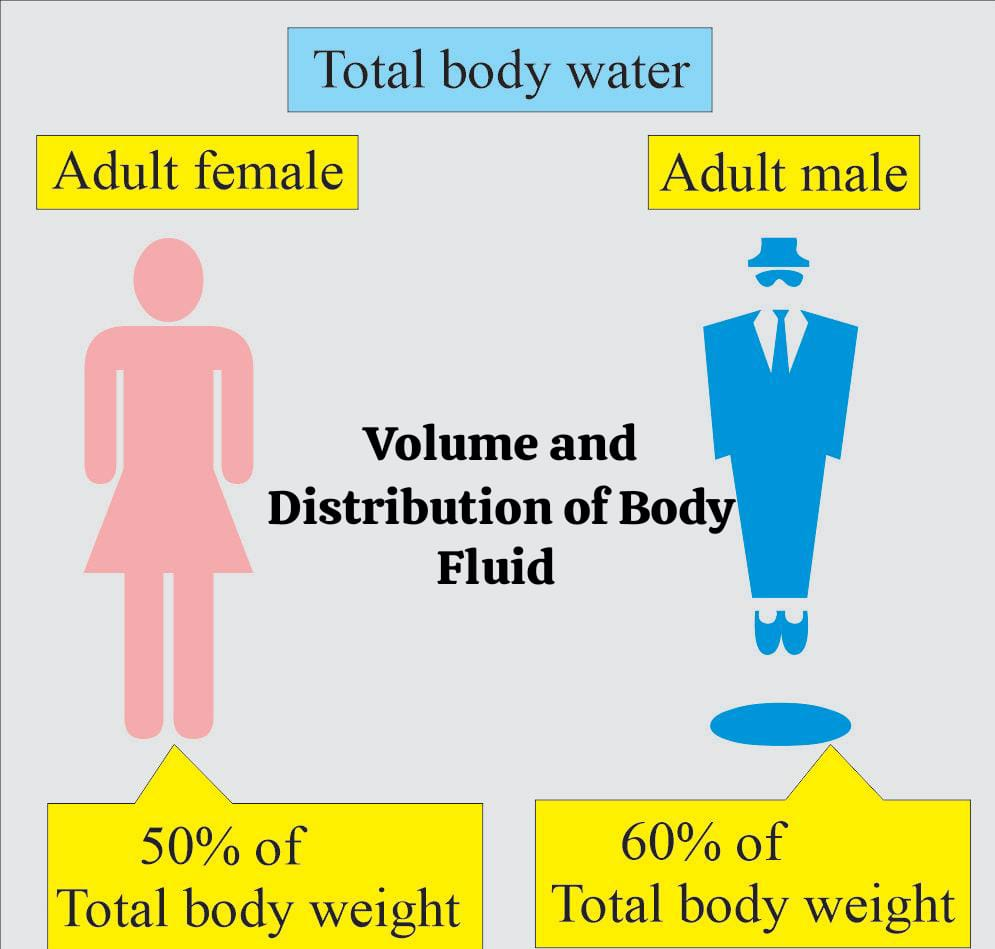
- The total amount of water that exists in the body at any given time is known as Total Body Water (TBW).
- Volume is approximately 60% of the body weight in adult men and 50-55% in adult women.
- Total body fluid is 60% of the body weight.
- Extracellular fluid is It is about 20% of body weight. Plasma (intravascular fluid) is 5%, interstitial fluid is 10-15% and transcellular fluid is 1 to 3%.
- Intracellular fluid is 40% of body weight.
Total Body Fluid as a Percentage of Body Weight in Relation to Age and Sex:
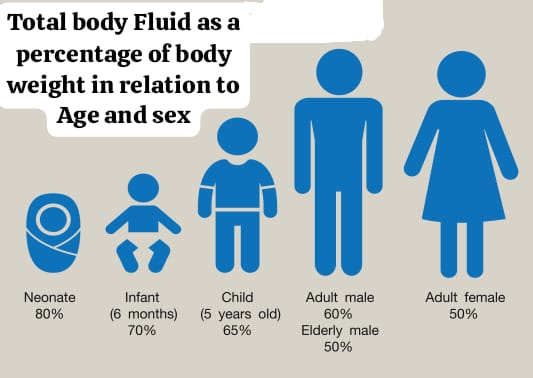
Age : Full term newborn
Total Body Fluid(% Body Weight): 40-80
Age : 1 Year
Total Body Fluid(% Body Weight): 64
AGE : Puberty To 39 Years
Total Body Fluid (% Body Weight) : Male: 60 & Woman: 52
Age : 40- 60 years
Total Body Fluid (% Body Weight): Man : 55, Woman: 47.
Age : More than 60 years
Total Body Fluid (% Body Weight): Man: 52, Woman: 46
Daily Fluid Requirement by Age and Weight:
Age: New born
Amount by weight (ml/kg): 60-100
Total amount (ml/24 hours): 200-300
Age: 6 months(6 months)
Amount by weight (ml/kg): 130- 140
Total amount (ml/24 hours): 900-1200
Age:1 year (1 year)
Amount by weight (ml/kg): 120- 140
Total amount (ml/24 hours): 1100- 3000
Age: 5 years(5 year)
Amount by weight (ml/kg): 100- 110
Total amount (ml/24 hours): 1500-2000
Age: 15 years( 15 years)
Amount by weight (ml/kg): 50-70
Total amount (ml/24 hours): 2200 – 2800
Age: Adult
Amount by weight (ml/kg): 20-30
Total amount (ml/24 hours): 2400-2600
Age: Above 65 years
Amount by weight (ml/kg): 20-50
Total amount (ml/24 hours): 2500-3000
Water is the main constituent of body fluids. It is very important to maintain a balance between water gain and loss. This balance can be maintained by roughly matching intake and output over a 24-hour period.
Fluid gain:

Fluid gain can be achieved through oral intake of fluids and food.
Average Fluid gain per day:
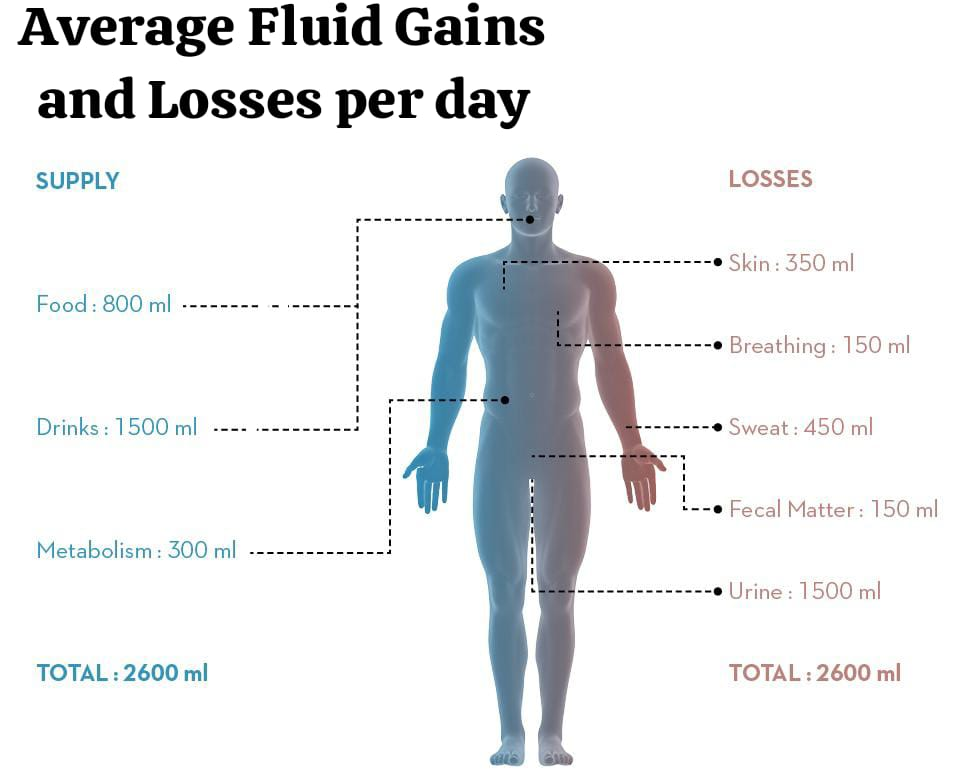
Ingested Fluid : 1200 -1500 ml.
Ingested Foods: 1000 ml.
Metabolic H2O : 300 ml.
Total (Total) : 2500 – 2800 ml.
Fluid loss :
1) Obligatory Loss: Important for the elimination of body waste.
2) Facultative Loss: Conserves water through urine or It depends on the body’s need to conserve or eliminate or regulate internal temperature.
3) Insensible loss: Insensible loss is moisture that continuously evaporates from the lungs and skin.
Average During the day class=”has-inline-color”>Fluid Loss ( Average Fluid losses per day):
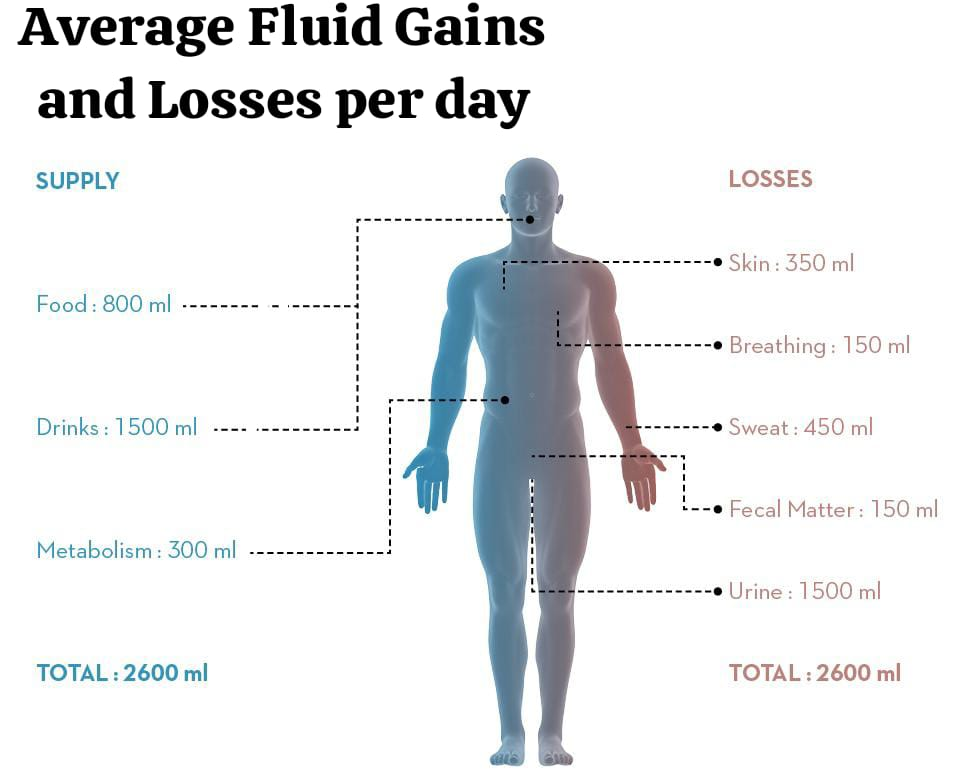
Urine: 1200-1500 ml.
Stool: 100 ml.
Lungs : 300-500 ml.
Skin: 600-800 ml.
Total: 2200-2900 ml.
Electrolytes:
Electrolytes are chemical compounds in a breakdown form when they are placed in solution. They carry electrical charges called ions. The ions dissolved in body water are called electrolytes. Acids, bases, and salts are electrolytes found in body fluids. Electrolytes are classified by the charge they carry.
Positively charged Electrolytes:
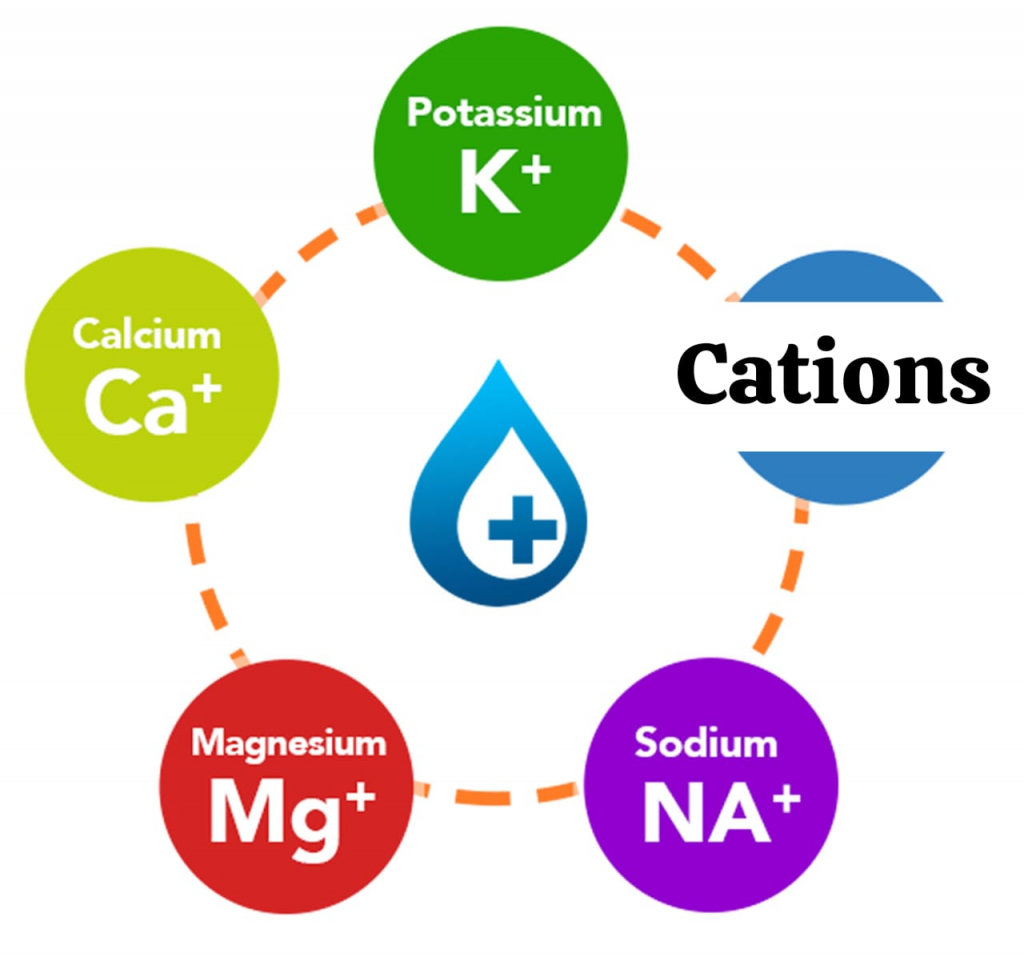
CATIONS:
- Sodium (Na+),
- Potassium(K+),
- Calcium( Ca2+),
- Magnesium (Mg 2+).
Negatively charged Electrolytes:
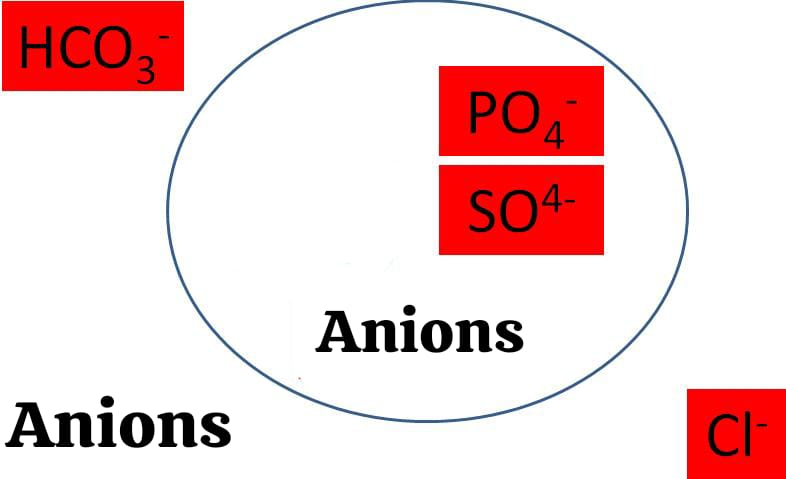
ANIONS:
- Chloride (Cl-)
- Bicarbonate (HCO3-)
- Phosphate (PO4-)
Factors Affecting Fluid and Electrolyte balance:
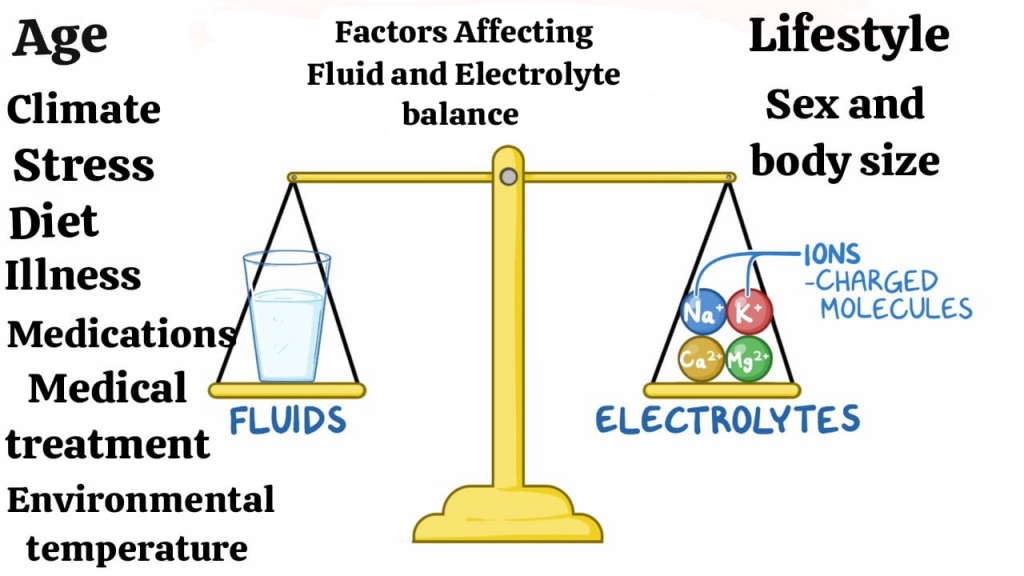
- Age (Age),
- Climate (Climate),
- Stress (Stress),
- Diet (Diet),
- Medication (Medications),
- Medical treatment (Medical treatment),
- Lifestyle (LifeStyle),
- Environmental Temperature (Environmental temperature),
- Sex and body size (Sex and body size).
1) Age:
At different ages, body weight, body surface area, renal filtration capacity, and metabolic rate differ, which influence fluid intake. Infants’ kidneys lose more fluid. Because the kidneys of infants are immature and are able to conserve less water compared to adult kidneys, the normal aging process in adults can affect fluid balance because the sensation of thirst decreases and the response of hormones that regulate fluid and electrolytes also decreases.
2) Climate:
Climate changes affect fluid intake and fluid loss. High heat and low environmental humidity increase sweating and fluid loss. Exercise, dry atmospheric conditions, heavy sweating can imbalance fluid and electrolyte balance.
3) Stress:
Stress stimulates the pituitary gland and releases antidiuretic hormone (ADH), which causes retention of water and sodium in the body and reduces urine output. Therefore, stress also plays an important role in the balance of body fluids.
4) Diet:
Intake of adequate amounts of fluids and nutrients is important to maintain fluid and electrolyte balance. Starvation causes the body to metabolize its own tissues for energy and leads to a decrease in available protein. When stored fat is used, ketones, strong acids, are produced and protein is used for the body’s energy. Albumin cannot be synthesized and can alter the ability to maintain intravascular osmotic pressure and fluid will shift from the intravascular to the interstitial space.
5)Illness:
Nausea, vomiting, diarrhea, increase metabolism, wounds and burns affect fluid and electrolyte balance. Knowledge about this condition will help to anticipate the need for fluid replacement.
6) Medication:
Excessive use of cathartics, enemas, diuretics, and steroids stimulate bowel evacuation by irritating the smooth muscles of the intestine. This can result in fluid volume deficit due to excessive water and electrolyte loss.
7) Medical Treatment:
Many medical treatments such as continuous gastric and intestinal suctioning, gavage feeding usually cause many imbalances along with persistent vomiting. Thus, medical treatment can lead to fluid and electrolyte imbalance.
8) Lifestyle:
Lifestyle factors such as diet, exercise, stress, and alcohol consumption affect fluid electrolytes and acid-base balance. People who have conditions like anorexia nervosa and bulimia nervosa are at risk of electrolyte and acid-base imbalance. Malnourished people have decreased protein intake, which can lead to edema (swelling) due to decreased oncotic pressure.
9) Environmental temperature:
In a hot environment, the risk of fluid and electrolyte loss through sweating increases.
10) Sex And Body Size:
Total body water is affected by sex and body size. Fat cells contain little or no water, but lean muscle tissue has a high water content. Women generally have more fat cells, so their water content is lower than men.
Alterations in Fluid and Electrolyte balance:

Many diseases and pathological conditions upset the normal fluid and electrolyte balance. Many diagnostic and therapeutic interventions can temporarily upset this balance.
Fluid volume alterations:
Fluid volume alterations include the following.
1) Fluid volume deficit.
2) Fluid volume excess Excess).
A) Fluid volume deficit:
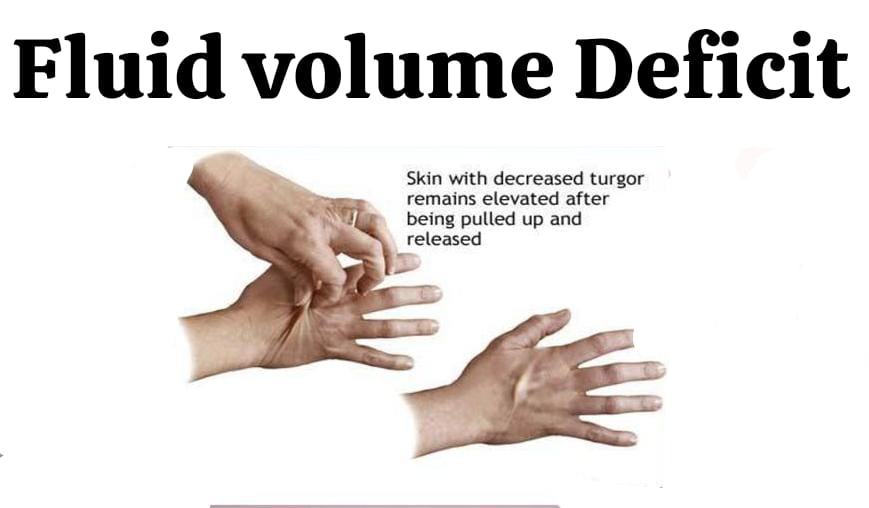
It is also known as dehydration and is defined as the loss of water and/or electrolytes from the extracellular fluid. Extracellular fluid is subject to proportional or disproportionate loss. Fluid volume deficit is a physiological situation in which fluid is lost in an isotonic fashion (proportional loss of water and electrolytes). Dehydration is the loss of water alone, resulting in a hyperosmolar state.
Disproportional losses: Two conditions exist. There are:
1)Hypotonic dehydration
2)Hypertonic dehydration
1) Hypotonic Dehydration:
Electrolyte loss is proportionately greater than loss of water (Electrolyte loss is proportionately greater than loss of water). Its causes include diabetes insipidus.
Diabetes insipidus
|
\/
Dilute urine loss from the kidneys due to decreased ADH (antidiuretic hormone) from the posterior pituitary gland.
|
\/
Dilute urine output.
|
\/
Extracellular fluid can leave the hypertonic fluid.
2) Hypertonic dehydration:
Water loss is Proportionately greater than electrolyte loss.
1)Hypovolemia:
Loss of blood volume due to hemorrhage or severe depletion of extracellular fluid from any other cause leads to circulatory failure.
2)Other effects include:
Brain cells are more sensitive. Tachycardia, restlessness, dry mucous membranes, poor skin turgor, decreased urinary output.
B) Fluid volume excess:

When water and solutes are taken in a proportionate amount from the extracellular fluid, it is known as extracellular fluid excess or overhydration.
Causes:
a) Rapid administration of IV fluids
b) Cardiac and Renal Failure
c) Liver Diseases.
Symptoms:
- Generalized edema,
- Wide spread accumulation of fluid in the interstitial spaces,
- Pulmonary congestion,
- Coughing, Dyspnea, moist Crackle on auscultation,
- Weight gain, jugular vein engorgement.
Acid – Base Alterations:
The body has sophisticated respiratory and renal mechanisms that protect against acid-base alterations.
1) Respiratory acidosis:
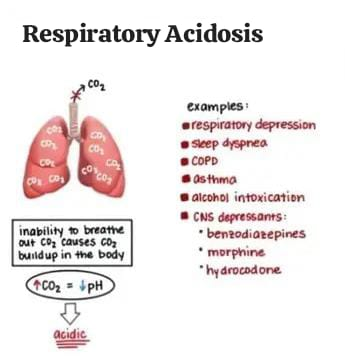
Respiratory acidosis is a condition that occurs when the lungs Not all carbon dioxide produced in the body can be removed from the body. Respiratory acidosis is a medical condition in which the ventilation of the body is reduced (hypoventilation) and the amount of carbon dioxide in the blood increases and the blood pH decreases. This condition is called acidosis.
Alveolar ventilation is reduced. In respiratory acidosis, the pH of the blood is less than 7.35. Pco2 is greater than 45 mmhg. This causes an acid-base imbalance and due to this, the body’s fluids and especially the blood become acidic. Carbon dioxide is produced as the body’s cells repair and carbon dioxide accumulates if sufficient carbon dioxide cannot be expelled from the body through alveolar ventilation. Then alveolar hypoventilation occurs. Due to this, the amount of paco2 in the body increases (hypercapnea).
Respiratory acidosis is also known as an excess of carbonic acid, it is dissolved in the blood There is an accumulation of carbon dioxide, which in combination with water forms an excessive amount of carbonic acid. Its causes include brain stem trauma, cerebral tumors, emphysema, asthma, and chronic bronchitis, which produce symptoms such as dyspnea, lethargy, and disorientation.
type of respiratory acidosis:
1) Acute respiratory acidosis
2) cronic respiratory acidosis.
1) Acute respiratory acidosis:
Acute respiratory acidosis occurs when ventilation suddenly fails. In Acute respiratory acidosis paco2 is Elevated above the upper limit of reference range( over 47 mmhg )( ph <7.35) .
Acute respiratory acidosis is caused by hypoventilation and is mainly due to disease of the central respiratory center located in the cerebral cavity, or due to any drugs, myasthenia gravis, Guillain-Barré syndrome etc. or may also be due to airway obstruction due to COPD (chronic obstructive pulmonary disease).
2) Chronic respiratory acidosis (Chronic respiratory acidosis):
Chronic respiratory acidosis can be due to pulmonary disease or any long-term disease or secondary to any other disease. In chronic respiratory acidosis, pCO2 increases above the normal range. Normal blood pH (7.35 to 7.45). Elevated serum Bicarbonate (hCO3- >30mm hg).
Chronic respiratory acidosis is caused by respiratory disorders, including COPD (copd:chronic obstruction pulmonary disease).
Chronic respiratory acidosis is secondary to obesity, hypoventilation syndrome, neuromuscular disorder, ventilatory defects and intestinal fibrosis, and thoracic deformity and is also seen due to lung disease.
cause/ Etiology (cause):
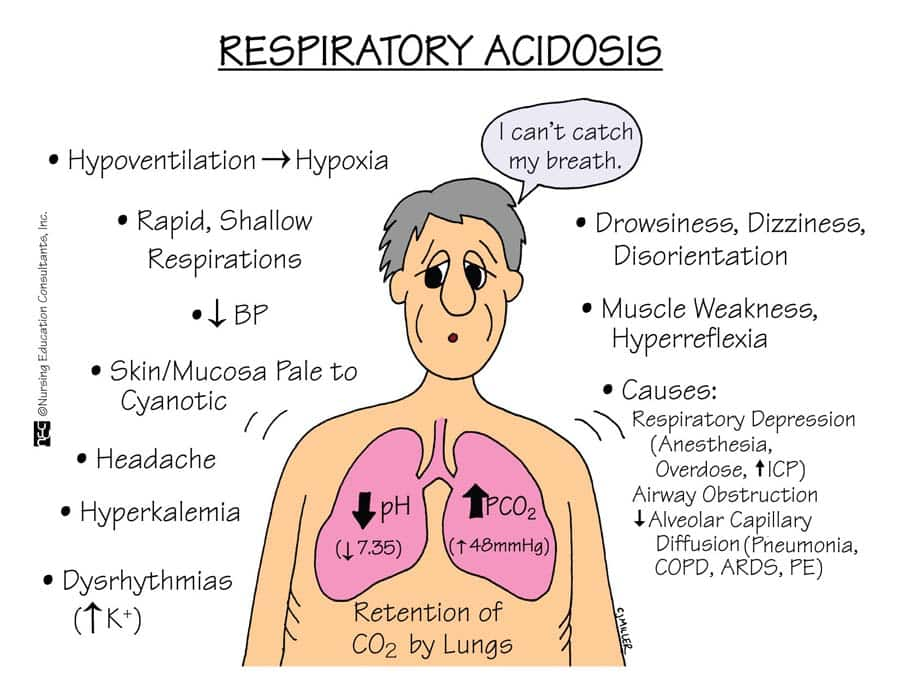
- drugs : narcotics,
- Anaesthetic,
- Hypnotics,
- Sedative etc,
- Trauma to the central nervous system such as medullary injury that impairs ventilatory drive.
- Airway obstruction.
- parenchymal lung disease.
- Chest wall disorder.
- severe kyphoscoliosis status post thoracoplasty.
- flail chest.
- alkalosis spondylitis.
- Chronic metabolic alkalosis that reduces alveolar ventilation.
- Neuromuscular diseases such as Guillain-Barre syndrome,
- Poliomyelitis In these diseases, the respiratory muscles cannot work properly, which causes hypoventilation.
- chronic obstruction Pulmonary disease ( copd),
- asthma,
- adult respiratory distress syndrome.
- chronic bronchitis.
- large pneumothorax.
- extensive pneumonia.
- Pulmonary Edema.
clinical manifestation/ sign and symptoms (ಲಕ್ಷಣೋ ಅನ್ನು ಚಿನ್ಹೋ):
- Difficulty in breathing,
- Shortness of breath,
- Fatigue,
- Chronic cough,
- Whizzing,
- Confusion,
- Irritability,
- Lethargy,
- Increase pulse rate,
- Increase respiratory rate,
- Increase blood pressure,
- Mental confusion,
- Felling of fullness in head,
- Anxiety,
- Delirium,
- Confusion,
- Papilloedema,
- Superficial blood vessels should be dilated.
Diagnostic evaluation
- History taking and physical examination,
- Arterial blood gas analysis.
- pco2 greater than 45 mmhg.
- pH is below the normal limit 7.35 to 7.45.
- Complete blood count tests.
- Monitoring of serum electrolyte level.
- chest X-Ray,
- Pulmonary function test,
- CT scanning,
- MRI of brain,
- fluoroscopy,
- ECG identify any cardiac involvement.
medical management:
- The goal of treatment is to correct the source of alveolar hypoventilation.
- Correct any disorder if present.
- Provide bronchodilator medicine to patient.
- Provide antibiotics medicine.
- Administer supplementary oxygen therapy.
- Advise to avoid smoking.
- Advise to lose weight.
- Provide Non invasive positive pressure ventilation.
- Dialysis to eliminate toxic drugs.
- Provide endotracheal intubation.
- Tracheostomy.
- Mechanical ventilation.
- Provide antibiotic medicine.
- PEEP : to prevent alveolar collapse.
- for Pulmonary emboli thrombolytics or anticoagulant therapy.
- Bronchoscopy to remove excessive secretion.
- administration B agonist like ipratropium, bromide, methylxanthines.
- Provide oxygen therapy and corticosteroids to the patient.
Nursing management:
- Monitor the patient’s arterial blood gas analysis.
- Assess the patient’s respiratory status.
- Provide the patient with a semi-recumbent position.
- Provide the patient with proper oxygen therapy.
- Listen to the patient’s breathing sounds.
- Ask the patient to do pursed lip breathing exercises.
- Ask the patient to ingest 300 ml of fluid.
- Provide the patient with a comfortable and workable environment.
2) define respiratory alkalosis:
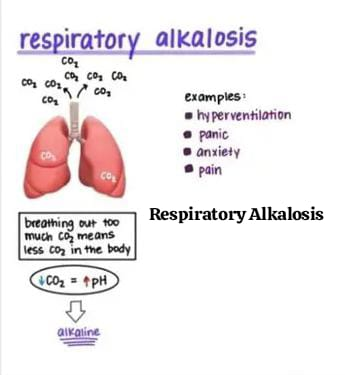
Respiratory alkalosis (respiratory Alkalosis is a condition in which the level of carbon dioxide is low due to excessive breathing and alveolar hyperventilation. In this, the level of partial pressure of carbon dioxide (pco2) is low (pco2 less than35 mmHg) and the blood pH is greater than 7.35.
Etiology/ cause:
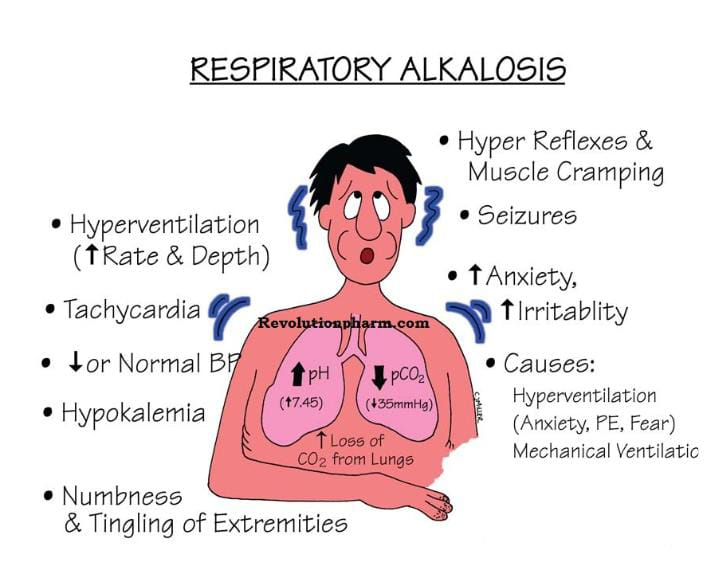
- Due to decrease PCO2 Level,
- Due to increase respiratory rate.
- Mechanical Ventilation ventilation).
- Pneumonia.
- Pneumothorax.
- Pulmonary edema.
- Pulmonary embolism.
- Emphysema.
- Acute asthma.
- Hyperthyroidism.
- Severe Anemia.
- Encephalitis.
- Hepatic failure.
- Hyperactive thyroid.
- Meningitis.
- Psychosis.
- Sepsis.
- Trauma.
- Tumor.
Clinical manifestation/ sign and symptoms:
- Signs and symptoms of respiratory alkalosis depend on its duration, severity and the extent to which the disease has progressed.
- Dizziness,
- Difficulty breathing,
- Numbness and tingling sensation,
- Numbness in the hands and feet,
- Disorientation of time, place and person.
- Confusion.
- Chest pain.
- Low chlorine levels in the blood (hypochloremia).
- Low sodium levels in the blood (hyponatremia).
- Paraesthesia (numbness and tingling sensation),
- Fainting.
- Syncope (a brief loss of consciousness when blood does not reach the brain or reaches it in small amounts).
- Tetany (muscle spasms).
- Breathing discomfort.
- Chest tightness.
- Tachypnea.
- Hand muscles spasm (Trousseau’s sign).
- Chovostek sign (facial muscles spasm when tapped on the front of the ear and cheek; this is seen in the body due to hypocalcemia).
Diagnostic evaluation:
- History taking and physical examination.
- Arterial blood gas level assessment.
- Blood culture.
- Brain MRI.
- Complete blood count.
- Liver function test.
- Serum analysis.
- Sputum culture.
- Urine culture.
- Chest X-ray.
- CT scan.
- Lumbar puncture.
- Cytologic analysis.
Medical management:
- The management of respiratory alkalosis involves treating the cause of hyperventilation.
- Hyperventilation can be treated by removing the ingested toxic agent such as salicylates,
- Using gastric lavage,
- Treatment of fever or sepsis,
- Oxygen administration,
- Asking the patient to do relaxation exercises, which can help reduce the patient’s anxiety level and improve breathing.
- Tidal volume and respiratory rate of a patient who is on mechanical ventilation are reduced.
- Perform arterial blood gas analysis of the patient.
- Give sedative and antidepressant medicine to the patient.
- If respiratory alkalosis is caused by anxiety, then sedative and tranquilliser medicine helps the patient.
- If the patient is in a hyper adrenergic state, then beta adrenergic blocker medicine should be provided to the patient.
Nursing management:
- Check the patient’s respiratory rate.
- Check the patient’s vital signs.
- Ask the patient to breathe slowly.
- Provide reassurance to the patient.
- Relieve the patient’s anxiety.
- If the patient is hyperventilating, then the patient’s anxiety level should be properly monitored and his respiratory level should also be checked.
- If the patient’s anxiety level is very high, then the patient should be counseled.
3) Metabolic acidosis:
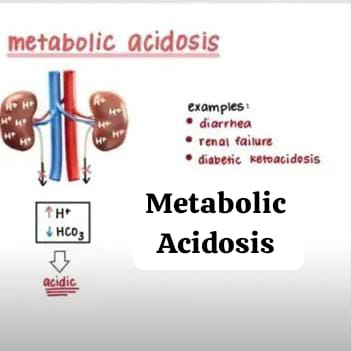
Metabolic acidosis is an acid-base disorder in which there is too much acid in the blood. It is present in the body fluid because the kidneys are not able to remove enough acid from the body and the concentration of bicarbonate in the body is low. Due to this, the condition of metabolic acidosis arises.
The condition of metabolic acidosis occurs when there is a lot of acid production in the body and the kidneys are not able to remove enough acid from the body, due to which the condition of metabolic acidosis arises.
Ex: blood pH is less than 7.35 due to the production of excess hydrogen ions in the body and the formation of bicarbonate (Hco3-) Due to low production.
Etiology/cause:
- Metabolic acidosis occurs when the body produces too much acid.
- When the kidneys do not remove adequate amounts of acid from the body.
- lactic acidosis,
- hyperchloremic acidosis,
- ketoacidosis,
- Kidney diseases ( distal tubular acidosis and proximal renal tubular acidosis),
- Chronic renal failure (due to accumulation of sulfate, phosphate and urea),
- poisoning by aspirin, glycol, or methanol,
- organic acid (salicylates ,thanol ,methanol, formaldehyde, glycol, paraldehyde and INH).
- Sulphate and metformin.
- Hypoaldosteronism.
- Lactic acidosis is caused by alcohol, cancer, liver failure, hypoglycemia and seizures.
- G.I.HCO3 loss due to diarrhea, fistula, ureterosigmoidostomy.
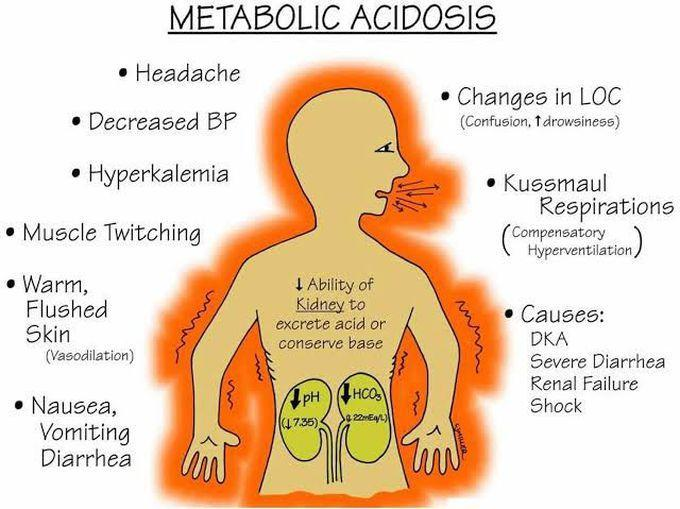
clinical manifestation sign and symptoms :
- Fatigue,
- Chest pain,
- Palpitation,
- Headache,
- Alter mental status,
- Severe anxiety,
- Loss of appetite,
- Impaired vision,
- Abdominal pain,
- Weight loss,
- Muscular weakness,
- Bone pain,
- Deep and rapid breathing,
- Increase in the amount of carbon dioxide,
- Fatigue,
- Drowsiness,
- Very sleepy,
- Visual disturbance,
- Retinal oedema
- vomiting,nausea,
- stupor.
Neurological:
- Lethargy,
- Stupar,
- Coma,
- Seizures,
- decrease cardiac output,
cardiac:
- Arrhythmias,
- Tachycardia,
- Hypotension: low blood pressure.
Diagnostic evaluation:
- History taking and physical examination.
- Arterial blood gas analysis to assess the severity of metabolic acidosis.
- Blood pH is less than < 7.35.
- Decrease the Bicarbonate level <24 mmol/liter.
- Metabolic panel to check the cause and severity of metabolic acidosis.
- Complete blood count.
- ECG will detect dysrrhythemias caused by the increased ketoacidosis.
- Elevation of ketone indicate diabetic, alcoholism, and starvation.
- Urine pH is less than 4.5.
- Serum potassium greater than 5.5 meq/liter and glucose level is greater than 150 mg/dl support the diagnosis of metabolic acidosis.
Medical management:
- Provide sodium bicarbonate 50 – 100 mmol to the patient to decrease the acidity of the blood and to neutralize the acidity in the patient whose blood pH is less than 7.20.
- If the metabolic acidosis is very serious, the patient should be put on dialysis.
- If the patient has hypokalemia, i.e. low amount of potassium in the body, then monitor the patient’s potassium level continuously and take measures to normalize it.
- Provide the patient with a mechanical ventilator to ensure proper breathing.
- Provide the patient with proper amount of fluid.
- Give the patient antibiotic medicine to protect him from infection.
- If the patient has decreased blood pressure, provide him with a drug to regulate it.
- If the patient has seizures, provide him with proper medicine.
- If the patient has nausea and vomiting, provide him with antiemetic medicine.
- Continuously monitor vital signs such as pulse, respiration, blood pressure and body temperature.
- Provide treatment to the patient as per the patient’s other needs.
Nursing care:
- Check the patient’s vital signs.
- Check the patient’s pulse.
- Assess the patient’s ECG pattern.
- Monitor the patient’s arterial blood gas analysis.
- Monitor the patient’s serum electrolytes, serum creatinine and blood urea nitrogen.
- Check the patient’s urine output hourly.
- Maintain the patient’s intake output chart.
- Check the patient’s heart sound, respiratory status and mental status.
- Conduct laboratory investigations of the patient.
- Check the patient’s level of consciousness.
- Maintain the patient’s intake-output chart.
- Check if the patient has seizures or any other condition.
- Provide the patient with a comfortable and functional environment.
4) Metabolic alkalosis:
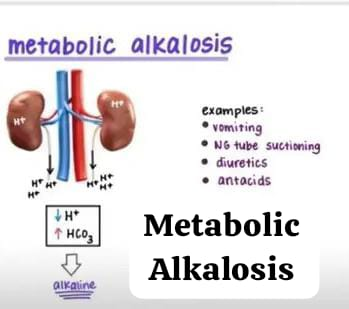
Metabolic alkalosis is a condition In which the concentration of bicarbonate (Hco3-) and the concentration of pH (ph) are high. This is due to the gain of bicarbonate (Hco3-) and the loss of hydrogen (H+) iron. One of the main causes of metabolic alkalosis is vomiting and gastric suctioning in which hydrogen and hydrogen ions are lost.
Etiology/ cause:
- Due to excessive accumulation of Hco3 (Bicarbonate).
- Vomiting,
- Alkali administration,
- Nasogastric tube drainage or lavage.
- Pyloric stenosis.
- Due to the use of steroid or diuretic medicines (furosemide, thiazide, and ethacrynic acid).
- External drainage of gastric fluid fluids).
- Due to prolonged ingestion of milk and calcium carbonate.
- Hypercorticism.
- Hyperaldosteronism.
- Cushing’s disease.
- Excessive ingestion of baking soda.
- Use of Antacid medication.
- Administration of excessive amounts of intravenous fluids with high bicarbonate concentrations.
- Blood transfusion.
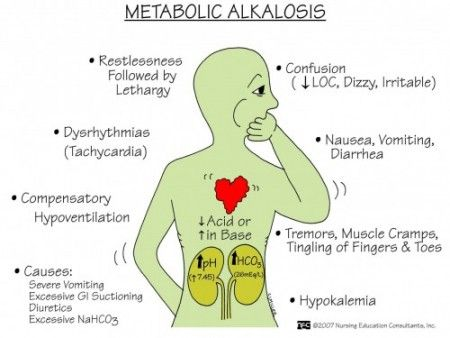
Clinical manifestation sign and symptoms:
- Headache.
- Dizziness.
- Fatigue.
- Tingling sensation in finger and toe.
- Hypertonic muscles.
- Neuromuscular excitability.
- Delirium.
- Tetany.
- Stunting.
- Difficulty in breathing.
- Tachycardia.
- Arrhythmias.
- Cyanosis: bluish discolouration of skin and nails.
- Nausea,
- Vomiting,
- Diarrhea,
- Irritability,
- Confusion,
- Increase heart rate,
- Irregular heart beat,
- Decrease blood pressure.
Diagnostic evaluation
- History taking and physical examination.
- Arterial blood gas evaluation with a pH level greater than 7.35.
- Serum bicarbonate concentration greater than 26 milliequivalents/liter (meq/liter).
- pco2 (partial pressure of carbon dioxide) level over 45 mmhg.
- Assessment of urinary chloride level.
- Serum electrolyte study shows low potassium,
Calcium And chloride level, - ECG finding sisclose alow t wave,merging with p wave,
Artirial and sinus tachycardia. - CT scan,
- MRI,
- Renal angiography
Medical management:
- Provide the patient with ammonium chloride (1 to 2 grams orally every 4 to 6 hours).
- Provide the patient with adequate chloride.
- Provide the patient with potassium chloride diluted in normal saline solution.
- Administer sodium chloride to normalize the patient’s fluid volume.
- Maintain the patient’s electrolyte balance normal.
- If the patient is receiving diuretic therapy, stop it.
- If the patient is hypokalemic, administer potassium chloride.
- If the patient has severe alkalosis, administer hydrochloric acid.
- Use carbonic anhydration to treat metabolic alkalosis.
- Administer hydrochloric acid intravenously at 0.1 to 0.2 mmol/kg/liter.
- Maintain proper intake and output of the patient.
- If the kidneys are not functioning properly, suggest dialysis to treat metabolic alkalosis.
- If the patient is nauseated and vomiting, provide antiemetic medicine.
- To monitor the patient’s vital signs properly.
- To provide the patient with proper fluid and electrolytes.
Nursing management:
- To monitor the patient’s respiratory rate rhythm and its depth.
- To check the patient’s level of consciousness.
- To assess the patient’s skin color.
- To maintain the patient’s electrolytes.
- To prevent the patient from complications (siezer, coma)
- To provide the patient with a safe and noise-free environment.
- To provide the patient with side rails.
- To administer the prescribed IV fluid to the patient.
- To check the patient’s arterial blood gas analysis and serum electrolytes.
- Check the patient’s vital signs and peripheral pulse.
- Check the patient for any condition such as phlebitis at the IV site.
- Provide the patient with proper oxygen.
- Check the patient for any muscle weakness, tetany, and decreased activity level.
- Maintain the patient’s intake output chart.
Electrolyte imbalance.
Sodium, potassium and calcium are the It plays an important role in the passage of urine. If any of these electrolytes increases or decreases, it causes muscle stimulation and electrolyte imbalance.
sodium imbalance:
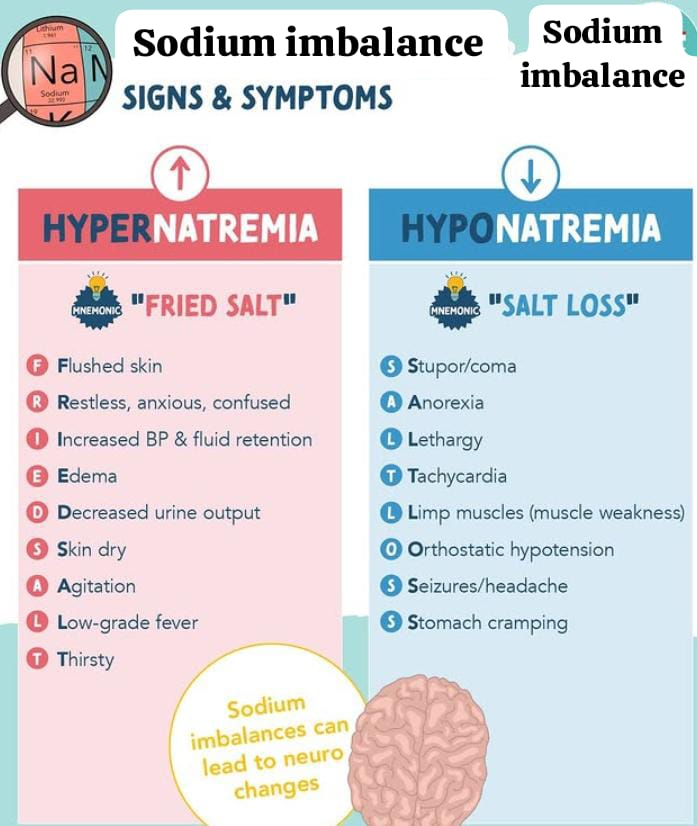
Sodium imbalance occurs when the concentration of sodium in the plasma is either too high or too low.
Normally, the concentration of sodium is 135 to 145 meq/liter.
sodium deficit/hyponatremia:
Sodium is an extracellular electrolyte. It plays an important role in water distribution. Sodium plays an important role in muscle contraction and the transmission of nerve impulses. The normal concentration of sodium in the blood is 135 to 145 meq/liter. Hyponatremia is a metabolic condition in which the level of sodium in the blood is lower than the normal sodium level.
(In hyponatremia sodium concentration is less than <135 meq/liter).
Three types of Hyponatremia:
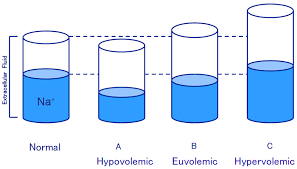
1) Euvolemic hyponatremia.
2) Hypervolemic hyponatremia.
3) Hypovolemic hyponatremia.
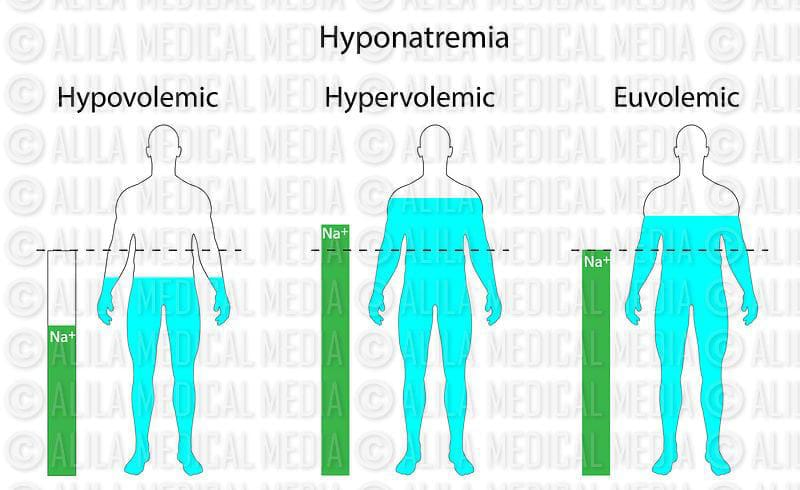
1) Euvolemic hyponatremia:
In this, the body’s water content increases. But the sodium level remains constant.
And this condition is caused by chronic health conditions, cancer and some medicines.
2) Hypervolemic hyponatremia:
In this, both water and sodium content in the body increases. But the amount of water is more. The excess water dilutes the sodium and reduces the sodium level. This is mainly due to kidney failure, heart failure and liver failure.
3)Hypovolemic hyponatremia.
In this condition, the level of water and sodium in the body decreases, but the level of sodium is lost more than water.
Etiology/cause of hyponatremia (Hypovolemic hyponatremia) Reason).
- Excessive intake of water during exercise
- Vomiting
- Hormonal imbalance
- Diarrhea
- Decrease amount of thyroid
- Syndrome of inappropriate Antidiuretic Hormone (SIADH)
- Due to increased thirst
- Due to nil per oral (NPO)
- Lithium therapy
- Diuretic
- Certain medications such as diuretics, antidepression, pain medications.
- Chronic or severe dehydration.
- Due to kidney failure.
- Due to kidney disease.
- Congestive heart failure.
- Burns.
- Hyponatremia may occur if you are on a low sodium diet.
Clinical manifestation/signs and symptoms of hyponatremia:
- Headache
- Muscle weakness
- Oligouria
- Vertigo
- Fatigue
- Restlessness
- Irritation
- Dizziness
- Lack of interest
- Muscle twitching and weakness
- Tachycardia (increased heart rate more than 100 beats/min)
- Nausea
- Vomiting
- Abdominal cramps
- Decrease urine output
- Postural Postural Hypotension
- Decrease Appetite
- Mental Confusion
- Delirium
- Coma
- Shock
- Confusion
- Stunned
- Cellular swelling with cerebral edema leading to headache
Diagnostic evaluation of the Hyponatremia:
- History taking,
- Physical Examination.
- Serum electrolyte level monitoring ex:sodium, Potassium, chloride.
- Serum sodium <135meq/liter. Urine specific gravity decreases. Decrease serum osmolarity. Urine sodium >100meq/24 hours.
Medical management of the Hyponatremia:
- Some hyponatremia conditions are caused by cancer. Radiation, chemotherapy, and surgery are provided to remove it so that the sodium imbalance can be corrected.
- Mild hyponatremia can be treated with diet, lifestyle, and medication.
- Severe hyponatremia can be treated with electrolyte and fluid administration.
- Intravenous solutions such as 0.9% normal saline fluid should be administered.
- Steroid therapy should be provided to reduce intracranial swelling.
- Check the patient’s intake output and daily weight.
- If the patient is lethargic, provide supplemental oxygen.
- Provide IV fluids as needed.
Nursing management of the Hyponatremia:
- Take a proper health history of the patient to determine the cause of the patient’s condition.
- Ask the patient to consume sodium-rich foods.
- Provide education to the patient to include sodium-rich fluids in the diet.
- If the patient’s sodium level does not increase by 12 meq/liter in 24 hours, administer Ringer’s latest and isotonic solution intravenously to the patient.
- Maintain an intake-output chart of the patient every 24 hours.
- Check the patient’s daily weight.
- Administer hypertonic normal saline to the patient.
- Check the patient’s level of consciousness.
- Check whether the patient is in a state of confusion, lethargy or not.
- Assess whether the patient is oriented to time, place and person or not.
- Assess the patient’s deep tendon reflexes, muscles tone and strength.
Prevention:
- Treat the condition that has caused the sodium level to be low early.
- Educate the patient who is taking diuretic medicine about the adverse signs and symptoms of taking diuretic medicine, e.g. hyponatremia.
- If the patient is exercising excessively, the patient should drink water only as needed to replace the fluid lost from the body through sweating and should not drink more than one liter of water in an hour.
- Ask the patient to eat sodium-rich foods.
- Ask the patient to eat a sodium replacement diet.
sodium excess/ hypernatremia:
Hypernatremia is an electrolyte imbalance in which The amount of sodium increases in the blood. When the amount of fluid in our body is less and if the salt is more and if the renal function is impaired, then the amount of sodium in the body increases and the condition of hypernatremia arises. The normal level of sodium in the body is 135 to 145 meq/liter. If the value of sodium in the body increases more than the normal value, it is called hypernatremia.
(In hypernatremia, the sodium level is >145 meq/liter).
Etiology/ cause of the hypernatremia(cause of hypernatremia):
- Due to loss of fluid from the body,
- Vomiting,
- Diarrhea,
- Sweating,
- High Fever,
- Dehydration,
- Due to inadequate intake of water,
- Certain medications such as steroids, licorice and certain blood pressure lowering drugs,
- Certain endocrine diseases such as diabetes and aldosteronism,
- Due to excessive salt intake.
- Due to excessive use of sodium bicarbonate.
- Due to uncontrolled diabetes.
- Due to heavy exercise.
- Due to renal dysfunction.
- Osmotic diuretics.
Clinical manifestation/sign and symptoms of Hypernatremia:
- Increase thirst.
- Dry and sticky mucous membranes.
- Restlessness and agitation.
- Decrease urine output.
- Decrease weight.
- Weakness.
- Firm tissues.
- Disorientation.
- Tachycardia.
- Confusion and personality change.
- Decrease consciousness level.
- Loss of appetite.
- Nausea.
- Vomiting.
- Fluid and Electrolyte imbalance.
- Pulmonary Edema.
- Pitting Edema.
- Abnormal Skin turgur.
- Postural hypotension.
- Difficulty in breathing.
- Diagnostic evaluation of hypernatremia:
- History Taking,
- Physical Examination,
- Serum electrolyte: serum sodium level >145meq/liter.
- High serum osmolarity.
- Increase urine specific gravity.
Management of hypernatremia:
- Infuse the patient with a hypotonic electrolyte solution intravenously.
- Instruct the patient to drink adequate amounts of water.
- Provide the patient with diuretics.
- Maintain the patient’s intake-output chart.
- Monitor fluid loss or gain in patients at risk of increased sodium levels.
- Maintain and assess the patient’s intake-output chart.
- Instruct the patient to eat a low-sodium diet.
- Notice the patient’s thirst.
- Check the patient’s body temperature for elevation.
- Check for alterations in the patient’s vital signs.
- Check the patient’s level of consciousness.
- Check the patient for headache, nausea, vomiting, and any changes in the patient’s vital signs.
- Monitor the patient’s intake-output chart and assess the sodium level.
- If the patient is having seizures, lower the bed and raise the side rails on the bed.
- If the patient has diabetes insipidus, ask him to drink plenty of water.
- Tell the patient to avoid salty foods, salt tablets, salty liquids, and soft drinks.
- Educate the patient to consume large amounts of water during exercise.
- Tell the patient to drink adequate amounts of water while taking diuretics.
- Assess the patient for increases or decreases in sodium levels.
- Maintain aseptic technique when providing intravenous fluids.
- Monitor the patient carefully if he or she has a fever or is vomiting uncontrollably.
- If the patient is vomiting profusely, admit him or her to the hospital immediately to prevent dehydration.
- Check whether the patient is oriented to time, place, and person.
- Provide reassurance to the patient.
Explain/Define of hypokalemia:
Potassium is a major intracellular It is a cation. About 98% of the body’s potassium is found inside cells. Potassium enters the cells during the formation of new tissues and during the conversion of glucose to glycogen. When such tissues break down, potassium leaves the cells and this can mainly be due to any trauma, dehydration or starvation. Potassium acts as an important electrolyte in the conduction of nerve impulses and the contraction of skeletal and smooth muscles.
Normal potassium levels are 3.5 to 5.5 meq/liter.
Hypokalemia is a metabolic disorder The condition in which the potassium concentration is lower than the normal potassium concentration is called hypokalemia.
In the condition of hypokalemia, the potassium level is less than 3.5 meq/liter.

( In hypokalemia potassium level is less than < 3.5meq/liter)
Etiology/cause of Hypokalemia (cause of hypokalemia):
- Due to Lack amount of potassium in diet.
- Due to high amount of potassium loss through kidney.
- Due to increase activity of aldosterone.
- Vomiting,
- Prolonged GI (gastrointestinal) suction.
- Due to increased perspiration.
- High amount of fluid loss from the GI tract.
- Increased potassium loss from diuretic medicine.
- Any trauma and fluid loss.
- Due to a tumor in the adrenal gland.
- Eating disorder.
- Diabetic ketoacidosis.
- Treatment of acidosis,
- Metabolic alkalosis,
- Aldosteronism or cushing syndrome.
- Renal tubular acidosis,
- Medication: potassium losing diuretic, digoxin and corticosteroids.
- Antibiotics: amphotericine B, carbenicillin and gentamicin.
Clinical manifestation/sign and symptoms of the Hypokalemia:
- Dizziness,
- Abnormal heart rhythm
- Fatigue.
- Lethargy
- Confusion
- Loss of appetite
- Nausea and vomiting
- Orthostatic hypotension (sudden dizziness when sitting or lying down).
- Cardiac arrest.
- Hyporeflexia
- Parasthesia
- Constipation
- Muscle weakness
- Pain
- Polyuria
- Nocturia
- Excessive thirst
- Respiratory distress
- Paralysis
- (Rhabdomyolysis: breakdown of muscles tissues).
Diagnostic evaluation of the Hypokalemia:
- History taking
- Physical examination
- serum potassium <3.5meq/liter
- Metabolic alkalosis
- 24 hour urine potassium excretion test.
- Ecg changes.
- test for kidney function (BUN and creatinine), glucose, Magnesium, And phosphorous.
medical management of the Hypolkalemia:
- If there is mild to moderate level of hypokalemia, then it can be removed by oral potassium supplement.
- If the patient has severe potassium deficiency, then he has to take potassium intravenously. Daily 40 to 80 meq/day.
- Ask the patient to take a diet rich in potassium orally.
Ex: Berries, bananas, strawberries, melons, organ milk, chicken, beans, broccoli, carrots, potatoes, raisins, raisins, dried grapes, all these foods should be taken which are rich in potassium. - If the patient cannot take it orally, then slowly infuse him with intravenous potassium chloride (KCL).
- Monitor the patient to see if he has an adequate amount of urine output.
- Check the patient for signs and symptoms of hypokalemia.
- Monitor ECG.
- Generally, the patient needs a low sodium diet and a high protein diet.
- Routine potassium intake for an adult is 50 to 100 meq/day in an average amount.
Nursing management of Hypokalemia:
- Take a proper health history and health assessment of the patient to find out the cause of the condition of hypokalemia.
- Administer potassium replacement therapy to the patient.
- Provide oral potassium by diluting it in proper water or juice.
- Check the IV site properly.
- Assess the patient’s intake output chart every hour.
- Check the patient’s vital signs.
- Check the patient’s heart rate and rhythm.
- Assess the patient for toxicity of any digitalis group medicine.
- Check the patient’s potassium level when the patient is taking diuretic medicine.
- Check the patient’s vital signs and pulse.
- Instruct the patient to eat potassium-rich foods.
- When administering potassium intravenously, do not infuse it too fast to maintain proper flow (not faster than 10-20meq/liter).
- Monitor the patient’s muscle tone and strength.
- Monitor the patient’s bowel sounds.
- Maintain the patient’s intake-output chart.
- Instruct the patient to consume adequate amounts of potassium in the diet to prevent loss of potassium levels.
Explain /Define of hyperkalemia:
Hyperkalemia is a condition in which In which the potassium level in the body or blood exceeds the normal potassium level.
Normal potassium level 3.5 to 5.5 meq/liter .
In hyperkalemia the potassium level is greater than 5.5meq/liter.
Etiology/causes of Hyperkalemia:
- Due to excessive use of potassium salts.
- Potassium intake in the body is high and the kidneys cannot excrete such amount of potassium from the body, due to excessive use of oral or intravenous potassium supplementation.
- Acute renal failure.
- Chronic renal failure.
- Adrenal gland insufficiency.
- Glomerulonephritis: Infection and inflammation of the filtering part glomerulus in the kidney is called glomerulonephritis.
- Metabolic acidosis.
- Rejection of kidney transplant.
- Lack of aldosterone.
- Addition disease.
- Type 1 diabetes.
- Burns.
- Hemolytic conditions.
- Rhabdomyolysis
- (breakdown of muscle tissue) from drugs, alcoholism, coma, or certain infection.
- Certain medications such as:
- ACE inhibitor,
- Potassium chloride,
- Heparin,
- Captopril,
- NSAID,
- Use of potassium sparing diuretic (ex:spironolactone).
Clinical manifestation/ sign and symptoms of Hyperkalemia.
- Irregular heart beat,
- decrease blood pressure,
- chest pain,
- arrhythmias,
- abdominal pain,
- nausea,
- vomiting,
- palpitation,
- muscle twitching or cramps.
- diarrhea.
- cardiac arrhythemia.
- ecg changes.
- muscular weakness and paralysis.
- confusion and coma.
- ventricular dysrhythmia and cardiac arrest.
- Respiratory failure.
- Tingling and numbness sensation.
- Cardiac arrest.
- Anemia.
- Muscle weakness.
Diagnostic evaluation of Hyperkalemia:
- History taking,
- Physical examination,
- serum potassium levels is (greater than)> 5.5meq.
- metabolic acidosis-serum pH falls below 7.35 .
- Ecg changes:
- Elevated T waves,
- Wide QRS complex,
- Prolonged pR interval,
- Flattened or absent p waves,
- Depressed ST segment.
Medical management of Hyperkalemia:
- Treatment of hyperkalemia depends on its cause, severity of hyperkalemia, and its symptoms, and the overall health of the patient.
- The patient should consume a low-potassium diet.
- Continuous cardiac monitoring of the patient should be done.
- The patient should be provided with proper intravenous fluids.
- Continuous ECG monitoring of the patient should be done.
- Medications that reduce the amount of potassium in the body should be provided to the patient.
- If the patient has severe hyperkalemia, dialysis is also necessary.
- If the amount of potassium does not decrease with medication, intravenous calcium gluconate should be administered to the patient.
- The patient should be provided with sodium bicarbonate to allow potassium to shift into the cells for a short time.
- The patient’s serum potassium level should be continuously checked.
- Medications that increase the amount of potassium in the body should be discontinued.
- The patient should be administered 25% or 50% glucose and insulin intravenously to allow potassium to re-enter the cells from the extracellular space.
- Intravenous calcium should be provided for a short time to reduce the effects of hyperkalemia and protect the heart and muscles.
- The patient should be provided with diuretic medications.
Nursing care for hyperkalemia:
- Assess the patient’s intake output chart.
- Administer potassium orally or parenterally.
- Avoid potassium-rich foods such as fruit juice.
- Assess the patient’s pain level and provide comfort measures.
- Assess the patient’s vital signs.
- Check the patient’s heart rhythm.
calcium imbalance:
Calcium is the most abundant mineral in the human body. It is critical and good for health. Normal calcium level in the body is 9 to 11mg/dl or 4.5 to 5.5 meq/liter.
Calcium is a mineral that is found in bones and teeth. More than 99% of calcium is found in bones. Calcium is an important component of bones and teeth. Calcium plays an important role in nerve impulses and contraction and relaxation of muscles, including cardiac muscles. Calcium plays an important role in blood clotting, muscle and nerve function.
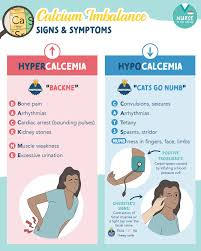
Explain/define hypocalcemia:

When there is not an adequate amount of calcium in the body and blood, it Hypocalcemia is called when the calcium level in the body is lower than the normal level.
Normal calcium level is 4.5 to 5.5 meq/liter, or 9 to 11mg/dl.
(In hypocalcemia the level of calcium in blood is less than 9 mg/dl or 4.5 meq/ liter. )
Etiology/cause of Hypocalcemia:
- The thyroid gland is underactive due to any disease or damage due to surgery.
- Due to excessive binding of calcium iron.
- Large amount of citrate in the blood.
- Hypoalbuminemia
- (Low albumin level).
- Alkalosis.
- Hyperphosphatemia (High amount of phosphate).
- Due to insufficient calcium intake in the diet.
- Due to inadequate vitamin D intake in the diet.
- Due to chronic renal failure.
- Due to inadequate absorption of calcium in the gastrointestinal tract.
- Intestinal fistula. (Fistula is an abnormal connection between two organs).
- Crohn’s disease,
- Chronic inflammatory bowel disease.
- Due to deficiency of parathyroid hormone and vitamin D.
- Severe burns or infections. Osteoporosis (this is a bone condition in which the bones are weak and fragile, which can easily break down).
- Due to infection of the pancreas.
- Kidney failure.
- Due to low amount of magnesium in the blood.
- Certain types of drugs such as diuretics, estrogen replacement therapy, glucose, calcium channel blockers, insulin and magnesium are responsible for the condition of hypocalcemia.
- Nutrition cannot be taken in adequate amount.
- Biphosphate therapy.
- Certain types of leukemia (blood cancer) and blood disorders.
- Toxic shock syndrome.
Clinical manifestation/ sign and symptoms of Hypocalcemia:
- Osteoporosis (bones become weak and fragile which can easily break down due to calcium deficiency).
- Anxiety and irritability,
- Pathogenic fracture (bones become weak due to any type of disease and therefore can easily break down).
- Tetany (tetany: muscle spasms due to calcium deficiency and underactive parathyroid gland).
- Tingling sensation around the nose and on the tips of the fingers.
- Tingling and numbness sensation also occurs in the hands and feet.
- Muscle spasms.
- Heart rate becomes irregular and increases.
- Nausea, vomiting.
- Blood pressure increases.
- Changes in mental status.
- Deep tendon reflexes become hyperactive.
- Gastro intestinal tract becomes enlarged.
- GI tract becomes enlarged, causing diarrhea and abdominal cramps.
- Cardiac arrhythmias.
- Laryngospasm (spasm of the vocal cords, causing difficulty in speech).
- Dry skin.
- Dermatitis (inflammation of the skin).
- Skin hyperpigmentation.
Chvostek sign :
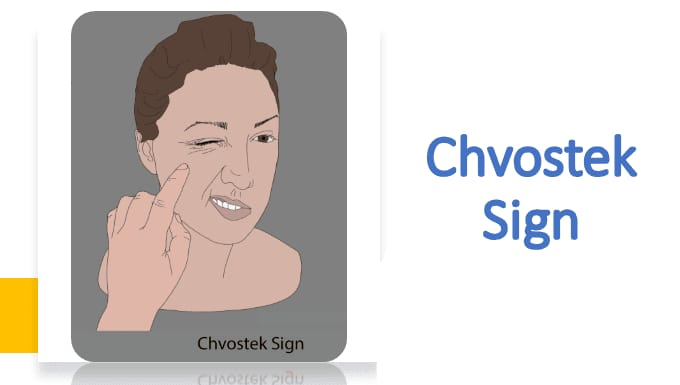
This sign occurs when the amount of calcium in the body is low. It is seen when there is an amount of it, in which when a person’s cheek and front of the ear are tapped, the facial muscles twitch.
Trousseau sign:
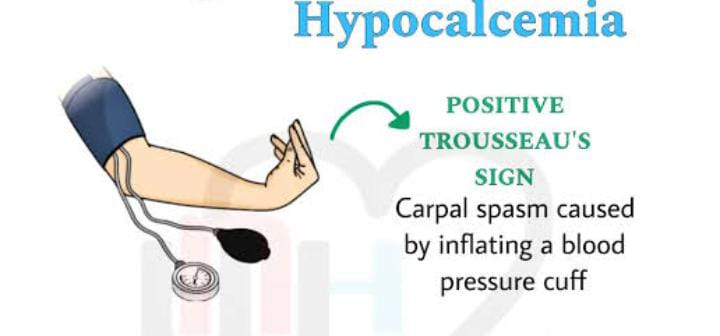
This sign is seen when there is a condition of hypocalcemia in the body. In it, when the blood pressure cuff is inflated to 20 mmHg more than the systolic blood pressure in a patient with hypocalcemia, carpopedal spasm is seen in which severe spasm occurs in the muscles of the hand. Neurological symptoms such as depression, personality change, seizures, lack of control in movement. Spasms occur in the muscles of the body.
Diagnostic evaluation of the Hypocalcemia (Hypocalcemia) Diagnostic Evaluation):
- History taking,
- Physical examination.
- Serum sodium level is less than<8.5 mg/dl.
- Low platelet count.
- Increase parathyroid hormone level.
- Ecg shows lengthened Qt interval, Prolong St segment, arrhythmias.
- Changes also occur in serum protein level as serum calcium is bound to albumin to some extent.
Medical management of the Hypocalcemia:
- Calcium supplementation should be given to the patient who has calcium deficiency.
- Vitamin D should be taken in proper amount.
- Calcium gluconate or calcium chloride should be given intravenously to the patient who has acute or severe hypocalcemia.
- Calcium supplements should be given one to two hours after meals to increase intestinal absorption.
- If the patient has severe hypocalcemia with cardiac arrhythmia and tetany, calcium salts should be given immediately.
- Calcium carbonate should be given in the initial stage of hypocalcemia.
- Calcium carbonate should be given in 250 or 500 mg/tablet.
- Calcium gluconate and calcium chloride should be given to the patient.
- If there is a serious condition, calcium chloride should be given to the patient.
- Calcium carbonate should be given in the initial stage of hypocalcemia.
- Vitamin D therapy should be taken in the proper amount.
- Consume vitamin D-rich foods such as milk or dairy products to ensure proper absorption of calcium.
Nursing management of the Hypocalcemia:
- Check the patient’s vital signs and blood pressure.
- Administer calcium intravenously to the patient.
- Monitor the patient’s airway and respiratory status.
- Provide education to the patient and his family members about sources of calcium.
- If the patient has undergone thyroid or neck surgery, carefully monitor the patient for signs and symptoms of hypocalcemia.
- Encourage the patient to exercise appropriately.
- Provide the patient with work and a quiet environment.
- Keep the patient’s bed at a low level and provide side rails.
- Ask the patient to avoid caffeine intake.
- Smoking reduces the amount of calcium in the body, so ask the patient to avoid smoking.
- Ask the patient to take a proper calcium-rich diet.
hypercalcemia:
Hypercalcemia is a condition in which the blood and body If the calcium level in the blood exceeds 11 milligrams per deciliter (11mg/dl) or 5.5 equivalents per liter (5.5 meq/liter), it is called hypercalcemia.
The normal level of serum calcium is (9 to 11 mg/dl or 4. 5 to 5.5meq/liter).
Hypercalcemia The level of calcium is greater than > 11mg/dl or 5.5 meq/liter).
Etiology/cause of Hypercalcemia Reason):
- Overactive parathyroid gland.
- Bone cancer causes excessive release of calcium from the bones.
- Dehydration, loss of water from the body.
- Multiple myeloma,
- Multiple fractures,
- Immobilization,
- Excessive calcium intake in the diet.
- Prolonged bed rest.
- Excessive absorption of calcium due to excessive amounts of vitamin D.
- Tumors that destroy bones.
- Excessive intake of calcium and vitamin D.
- Excessive consumption of milk products.
- Chronic kidney failure.
- Alkalosis.
- Dietary excessive intake of calcium.
- Due to increased absorption of calcium due to vitamin D.
- Chronic kidney disease.
- Due to the use of certain medications such as thiazide diuretics.
- Inherited kidney or metabolic conditions.
Clinical manifestation/sign and symptoms of the hypercalcemia:
- Increased heart rate.
- Increased blood pressure.
- Muscle weakness.
- Loss of appetite.
- Bone pain and pathological fractures.
- Constipation.
- Vomiting.
- Abdominal pain.
- Body ache.
- Decreased ability of blood to clot.
- Nausea and vomiting.
- Passing excessive urine (polyuria) and feeling very thirsty (polydipsia).
- Feeling tired (lethargy).
- Kidney stones form and waste products build up in the body.
- Confusion.
- Heart block.
- Difficulty speaking.
- Very sleepy.
- Headache.
- Irritability.
- Depression.
- Memory impairment.
- Mood swings.
- Confusion.
- Renal stones.
- Decreased deep tendon reflexes.
- Coma.
Diagnostic evaluation of the Hypercalcemia:
- History taking
- Physical examination.
- Serum calcium level is greater than 10.5 mg/dl.
- ECG shows sign of heart block. Shortened QT interval and ST segment.
- X-ray may reveal the presence of osteoporosis, bone cavitation or urinary calculi.
- Urine analysis.
- Decreased parathyroid hormone.
- Sulkovich urine test shows increased calcium precipitation.
Medical management of the Hypercalcemia:
- If the patient has a condition of severe hypercalcemia, then he needs to be hospitalized immediately.
- Patients who have a condition of severe or acute hypercalcemia should be provided with normal saline and their hydration status should be maintained.
- Patients who have nausea, vomiting, polyuria should be provided with normal saline.
- Provide Lasix (Frusemide) to patients who are taking diuretic medications to increase the amount of calcium excreted from the body.
- Use medications that bind calcium and release it from the body.
- Ask the patient to undergo embolization.
- Advise the patient to drink plenty of fluids.
- Provide reassurance to the patient and his family members.
- Biphosphates help in the absorption of calcium into the bone.
- Administer glucocorticoids.
- Dialysis.
Nursing management of the Hypercalcemia:
- Maintain the patient’s intake output chart.
- Ask the patient to take adequate amounts of fluids.
- Advise the patient to follow a low calcium diet.
- Provide reassurance to the patient’s family members.
- If the patient’s serum calcium level increases above 5.5 meq/liter, check the patient for cardiac arrhythmias.
- If the patient is using diuretics or normal saline, check the patient for signs and symptoms of heart failure.
- If the patient is receiving glycosides, check for toxicity such as loss of appetite, nausea, vomiting, bradycardia, etc.
- If the patient has bone weakness, carefully change the patient’s position.
- If the patient is bedridden, change the patient’s position frequently and ask the patient to do range of motion exercises.
- Check the patient’s vital signs.
- Assess the patient’s respiratory status.
- Check the patient’s heart sound.
- Ask the patient to do physical activity to maintain weight.
- Ask the patient to eat adequate amounts of fiber-rich food to relieve constipation.
- Ask the patient to eat a low calcium diet and increase fluid intake.
- Ask the patient to do activities so that calcium can be reduced from the body.
Introduction of magnesium:

Magnesium Essential Component Magnesium is an essential mineral that regulates over 300 enzymes that are responsible for many body functions. Magnesium works as a cofactor for many body enzymes. Magnesium plays an important role in the body’s metabolic activity. Magnesium is involved in the relaxation of smooth muscles, such as those around the bronchial tubes, and the contraction of skeletal muscles and neurons in the brain. Magnesium helps in protein buildup and maintains calcium levels in the blood. Magnesium prevents cardiovascular diseases and regulates irregular heart beats. Magnesium helps prevent the condition of heart attack. Magnesium prevents the condition of stroke.

(The normal value for serum magnesium is 1.5 to 2.5 meq/liter ) or (1.8-3.0mg/dl)
If there is an imbalance in magnesium, it is called hypomagnesemia (Hypomegnesemia) or hypermagnesemia (Hypermegnesemia).
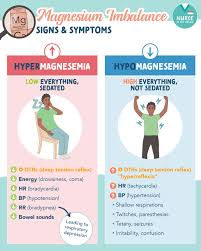
Magnesium deficit (hypomagnasemia)(hypomagnesemia):
Hypomagnesemia is an electrolyte disturbance in which the level of magnesium in the blood is abnormally low, less than the normal level of magnesium.
(The normal adult value of magnesium is 1.5-2.5meq/liter.)
(In hypomagnesemia the level of Magnesium is less than <1.5 meq/liter)
Etiology/cause of Hypomagnesemia Reason for):
- Magnesium is lost in large quantities from the body.
- Due to low intake of magnesium.
- Due to protein-calorie malnutrition.
- Due to improper absorption in the intestine.
- Due to malnutrition or starvation.
- Due to excessive fluid loss from the body.
- Due to excessive urine excretion from the body.
- Severe diarrhea.
- Crohn’s disease.
- Due to excessive diuretic administration.
- Use of certain medications including amphotericin,
- Cisplatin, Aminoglycoside.
- Gastrointestinal fistula.
- Renal damage.
- Total parenteral nutrition.
- Excessive urination (polyuria).
- Alcoholism.
- Malabsorption.
- Due to high blood calcium levels in the body.
- Hyperaldosteronism.
Clinical manifestation/sign and symptoms of Hypomagnesemia:
- Positive Trousseau’s and Chovostek signs.
- Loss of appetite.
- Nausea.
- Vomiting.
- Weakness.
- Apathy.
- Muscle weakness.
- Parasthesia.
- (tingling or numbness sensation).
- Movement becomes slow and involuntary.
- Irritability,
- Tetany,
- Leg and foot cramps.
- Staggering.
- Tremors,
- Ataxia: Lack of normal coordination.
- Carpopedal spasm (spasm of the hand muscles).
- Depression.
- Irritability.
- Psychotic behavior.
- Staggering.
- Vertical nystagmus: Involuntary movement of the eyeballs.
- Cardiac dysrhythmias.
- Extreme agitation.
- Insomnia.
- Delirium.
- Auditory and visual hallucinations.
Diagnostic evaluation of the Hypomagnesemia:
- History taking.
- Physical Examination.
- Serum Magnesium lower than 1.5 meq/liter.
- Nuclear Magnesium Resonance Spectroscopy.
- Electrocardiogram shows:
- A) Prolonged PR and QT intervals,
- B) Widening QRS,
- c) ST segment depression.
- D) Flattened T waves.
- E) Prominent U wave.
Medical Management of Hypomagnesemia:
- Treatment of hypomagnesemia is provided based on the severity of the deficiency and the clinical effects.
- The aim of treatment is to identify and eliminate the cause of hypomagnesemia and replace the amount of magnesium in the body.
- Provide oral magnesium replacement to patients with mild symptoms.
- Provide intravenous magnesium replacement to patients with very severe signs and symptoms and advise patients with mild hypomagnesemia to eat a magnesium-rich diet.
- Like: Green leafy vegetables,
Nuts,
Legumes,
Whole grains,
Sea food, etc.
Take magnesium preparations such as magnesium oxide as they contain high amounts of magnesium. - Provide intravenous magnesium sulfate to patients with severe hypomagnesemia. (10 to 40 meq/liter).
- If the patient has conditions like cardiac arrhythmia,
Obstetrics problem,
Electrolyte disturbance,
Asthma.
etc., provide intravenous magnesium sulfate.
Nursing management of the Hypomagnesemia:
- Ask the patient to take magnesium rich food.
- Monitor the patient’s level of consciousness.
- Monitor the patient’s breathing pattern.
- Assess whether there are changes in the patient’s E.C.G. Assess Ecg changes in a person who is taking digitalis group of medicines.
- Advise the patient to avoid excessive amounts of diuretics and laxatives.
- Monitor the patient’s bowel sounds and abdominal distension.
- Check the patient’s reflexes before infusing magnesium.
- Provide the patient with a quiet, dark room to work in.
- Provide the patient with a low bed and raise the side rails to prevent falls if the patient is hesitant.
hypermegnesemia:
Is hypermagnesemia an electronic imbalance? In which the magnesium level in the body increases to 2.5 meq/liter.
A normal level of magnesium in the body is important for the function of the heart and nervous system.
(In hypermagnesemia the level of magnesium is greater than > 2.5 meq/liter).
Etiology/cause of the Hypermagnesemia:
- Hemolysis,
- Renal insufficiency,
- Excessive magnesium intake due to overuse of antacids and laxatives.
- Hypothyroidism.
- Lithium therapy.
- Diabetic ketoacidosis.
- Adrenal insufficiency.
- Overdose of magnesium salts.
- Severe dehydration.
- Chronic renal insufficiency.
Clinical manifestation / sign and symptoms of the Hypermagnesemia:
- Nausea and vomiting,
- Weakness,
- Difficult breathing,
- Low blood pressure (hypotension),
- Bradypnea,
- Fatigue,
- Drowsiness,
- Skeletal muscle weakness,
- Diminished reflexes,
- Facial paresthesia,
- Flaccid muscle paralysis,
- Hypercalcemia,
- Arrhythmia,
- Depressed deep tendon reflexes.
- Bradycardia.
- Slow breathing.
- Bradycardia.
- Cardiac failure.
Diagnostic evaluation of the Hypermagnesemia:
- History taking,
- Physical examination,
- serum Magnesium level is greater than 2.5 meq/liter.
- Elevated potassium and calcium levels.
- ECG changes: prolonged PR interval ,
- Tall t waves,
- Prolonged QT interval and Widened QRS.
- Medical management of the Hypermagnesemia:
- Instruct the patient to take a large amount of fluid.
- Provide the patient with diuretic medicine.
- Infuse calcium gluconate to prevent magnesium toxicity.
- If the patient is unable to pass magnesium through urine i.e. if his kidney function is impaired, then provide him with diet.
Nursing management of the Hypermagnesemia:
- Monitor the patient carefully.
- Maintain the patient’s intake output chart.
- Tell the patient not to take magnesium rich food.
- Monitor the patient for lethargy or drowsiness.
- Avoid over the counter drugs that contain magnesium.
- Check the patient’s vital signs.
- Assess the patient’s respiratory function.
- monitor Ecg changes like:( prolonged PR, prolonged QRS, and prolonged QT.)
- Advise the patient to consume foods low in magnesium.
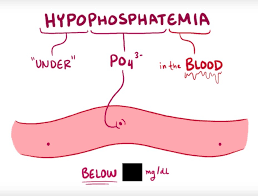
hypophosphatemia:

Hypophosphatemia is a condition in which In which the level of phosphate in the body is lower than the normal level. Hypophosphatemia is caused by starvation and alcoholism.
(normal Phosphate level is 2.5 to 4.5 mg/dl( 0.8 -1.5 mmol/liter))
In mild hypophosphatemia ( 2-2.5mg/dl or 0.65 -0.81 mmol/liter),
In moderate Hypophosphatemia( 1-2mg/dl or 0.32 -0.65 mmol/ liter),
In severe hypophosphatemia(<1mg/dl or 0.32 mmol/liter).
Etiology/ cause:
- Administering intravenous fluid without phosphorus.
- Hyperparathyroidism: This is a condition in which the parathyroid gland secretes too much parathyroid hormone.
A condition called hypophosphatemia may arise due to poor kidney function in which the kidney tubules are unable to reabsorb adequate amounts of phosphorus. - Recovery from certain diseases such as diabetic ketoacidosis (diabetes ketoacidosis) or severe burn can cause hypophosphatemia.
- Due to excessive use of diuretic medicine.
- Due to malnutrition due to long-term alcoholism and inadequate intake of phosphorus.
- Due to any intestinal abnormality in which the intestine cannot absorb phosphorus in an adequate amount. Ex:chronic diarrhea.
clinical manifestation/(sign and symptoms):
- loss of appetite,
- weakness in skeletal and smooth muscles.
- tingling sensation sensation).
- Numbness.
- Paresthesia ( feeling of tingling and numbness sensation).
- Tremors.
- Bone pain.
- Weakness.
- Respiratory Respiratory insufficiency.
- Impaired neurologic function.
- Confusion.
- Memory loss.
- Stunned.
- Coma.
- Hemolytic Anaemia.
- Impaired leukocytes and platelet count.
Diagnostic evaluation:
- History tacking (history tacking)
- Physical examination (physical examination).
- Serum Phosphate level is less than 2.5 mg/dl.
- urin Phosphate level greater than 1.3 g/24 hours.
medical management:
- Advise the patient to include phosphate in their diet.
- Provide medication supplementation to the patient if phosphate replacement through diet is not achieved.
- Provide intravenous phosphate to the patient if medication is not successful.
- Advise the patient to take phosphate rich food.
Ex: green leafy vegetable 🥦,
peas
beans
nuts,
chocalate,
beef liver,Etc.
Tell the patient to take phosphate rich food. - If the patient has severe hypophosphatemia, then provide intravenous phosphate.
- the dose of kpo4 2.5 mg/dl every 6 hourly.
- If If the patient has hyperparathyroidism, the parathyroid gland should be surgically removed.
- Nursing management:
- Check the patient’s serum electrolyte level.
- Instruct the patient to include phosphate-rich foods in his diet.
- Check the patient for muscle weakness.
- The patient’s Check mental status.
- Check the patient’s vital signs.
- Maintain the patient’s intake output chart.
hyperphosphatemia:

Hyperphosphatemia is a condition in which the level of phosphate in the body and blood increases above normal levels. Hyperphosphatemia is caused by excessive absorption of phosphate, decreased loss from the body, and excessive production. One of the main causes of hyperphosphatemia is renal failure.

Etiology/cause:
- Renal failure,
- Chemotherapy,
- Hypothyroidism ),
- Respiratory acidosis,
- Diabetic ketoacidosis,
- Due to excessive intake of phosphate.
- Profound muscles necrosis.
- Hypophosphatemia is a condition caused by excessive use of laxatives and enemas containing phosphate. It is caused by.
- Posphate is not excreted from the body due to kidney failure and magnesium deficiency.
- Hypoparathyroidism is a condition in which the parathyroid gland does not produce adequate amounts of parathyroid hormone.
- It is caused by the inability of the kidneys to respond to parathyroid hormone, which results in the body not excreting phosphate.
And the amount of phosphate in the body Increases. - The amount of phosphate in the body also increases due to the condition of hypocalcemia.
clinical manifestation/sign and symptoms:
- Tetany,
- Tingling sensation in the fingertips.
- Loss of appetite.
- Nausea.
- Vomiting,
- Muscle weakness.
- Hyperreflexia.
- Tachycardia.
- Decrease in urine output.
- Blurred vision.
- Palpitations.
- Depression.
- Loss of memory.
- Convulsions.
- Calcification of soft tissues.
- Arteriosclerosis.
- Arteriosclerosis can lead to heart attacks and strokes.
- Severe itching.
Diagnostic evaluation:
- History taking and physical examination.
- Serum phosphate level is greater than 4.5 mg/dl.
- Serum calcium level is less than 9 mg/dl.
- X-ray,
- Decrease parathyroid hormone,
- Check blood urea nitrogen level (BUN) and creatinine level.
Medical management:
- Provide vitamin D preparation to the patient to reduce phosphate level.
- Use oral phosphate binders that can absorb phosphorus from the G.I track.
- (Lanthanum carbonate provides to the dialysis patients). lanthanum carbonate provides to the dialysis patients.
- Use calcium salts such as (calcium carbonate, gluconate, and chloride) that bind phosphate from the blood stream and reduce the level of phosphate in the blood.
- Use albumin salts such as albumin hydroxide that can bind phosphorus.
- If the patient has severe hyperphosphatemia, calcium gluconate should be provided intravenously to the patient.
- Dialysis is an important treatment to remove excess phosphate from the blood.
- Foods rich in phosphate, such as dairy products, meat, nuts, and other high-protein foods, should be avoided.
Nursing management:
- Assess the patient’s serum phosphate level.
- Check the patient for signs and symptoms of hypocalcemia.
- Monitor patients who are at high risk for hyperphosphatemia properly.
- Instruct the patient to follow a low-phosphate diet.
- Instruct the patient to avoid phosphate-rich foods such as cheese, cream, nuts, whole grains, cereals, dried fruits, vegetables, sweetbreads, and milk foods.
- When the patient’s phosphate level is normal, instruct the patient to avoid laxatives and enemas that are high in phosphate.
- Instruct the patient to check for any signs and symptoms of hypocalcemia and assess the patient for any changes in urine output.
- Advise the patient to eat a low-phosphate diet.
Nursing measures to promote optimum Fluid and Electrolyte balance:
Preventive care:
In this, the client is given the reasons for fluid and electrolyte imbalance so that they can take preventive measures.
a)Life style analysis :
By analyzing factors such as the client’s age, stress, diet, and electrolyte imbalance can be prevented.
b)Life style counseling
C) Health Education (health Education):
Stress relieving factors such as dietary pattern and medical symptoms (Dietary pattern and medical symptoms) e.g. :
Hyperirritability,
Bradycardia,
Shallow Respiration,
Parasthesia.
Common Laboratory test to Assess Fluid, Electrolytes and Acid – Base Balance:
1)Serum Osmolarity(Serum Osmolarity):
Measures the total concentration of dissolved particles in serum; Mostly determined by Na+ concentration.
Normal Findings:
Child:70- 290 mosm/kg,
Adult:280- 300 mosm/kg.
2) Urine Osmolarity:
Urine Osmolarity Measures the concentration or number of solute particles, regardless of size.
Normal Findings:
Newborn:100-600 mosm/kg
Child/Adult: 50-1200 mosm/kg
Average:200 – 800 mosm/kg.
3) Urine Specific Gravity Gravity):
The density of water is not as precise a measurement as urine osmolarity in comparison to distilled water.
Normal Findings:
Urine Specific Gravity:1.003-1.040
4) Hematocrit (Hct ( Hematocrit)):
Measuring the percentage of red blood cells by volume in the whole blood provides a relative indication of fluid volume alterations.
Normal Findings:
Adult Male: 40-54%
Adult Female: 36-46%
5) Hemoglobin (Hgb or Hg):
Measures the oxygen carrying capacity of the blood; It is an indicator of fluid balance.
Normal Findings:
Adult Male: 14-18 gm/dl
Adult Female: 12-16 gm/dl
6)Blood Urea Nitrogen (BUN: Blood urea nitrogen):
It measures the level of nitrogenous waste in the bloodstream. Relative indicators are affected by factors such as the amount or decrease of protein in the diet, catabolic drugs, and growth spurts in children.
Normal Findings:
6-20 (mg/dL) or
2.1-7.1 (mmol/L).
The client should be treated to maintain fluid and electrolyte balance in the body.
d) Health Screening:
The client must undergo all laboratory tests to check the fluid and electrolyte balance in the body.
Supportive care:
1) Diet and Fluid Counseling:
Encouraging the client to have a proper and regular diet and fluid intake, which will Fluid and electrolyte balance can be achieved.
2) Oral Rehydration:
Encourage the client to take oral fluids which increases fluid and electrolyte balance.
Electrolyte balance tests:
1)Serum Electrolytes: Measures the electrolyte balance in blood serum.
Sodium (Na+) : 135-145 mEq/L
Potassium (K+) : 3.5-5 mEq/L
Calcium (Ca2+): Ionized : 4.5 – 5.8 mEq/L
Total : 8.8 – 10.5 mEq/L
Magnesium (Mg2+): 1.5-2.5 mEq/L.
Phosphorus (PO42-):2.5-4.5 mEq/L
Chloride (Cl-): 98-108 mEq/L
2) Urine Electrolytes(Urine Electrolytes):
Measures electrolyte levels in urine; 24-hour urine collection is involved.
Sodium (Na+) : 40-220 mEq/L per 24 hours
Potassium (K+) : 40-80 mEq/L per 24 hours
Calcium (Ca2+) : 50-300 mEq/L per 24 hours
3) Balance Test: Gases (ABGs) :
PO2 : 80 – 100 mmHg
PCO2 :35-45 mm Hg
pH : 7.35-7.45
HCO3 : 22-26 mEq/L
Base excess : +2 to -2 mEq/L
Fluid loss :
Current Body Weight ×60 % = Normal Na+( Normal Na+)/
Current Na+( Current Na+)
Limitations of Oral Fluid Intake:
Unconscious client,
Mouth trauma
Swallowing difficulty,
Indigestion
Intake output monitoring and Recording:
There should be a proper chart for monitoring and recording intake and output.
Indication Of Recording Intake and Output:
- Acute and chronic diseases (renal, cardiac, endocrine, neurological, hepatic, dermatological),
- Major trauma, surgery, draining wounds, altered intake or output.
- Insufficient diet, nausea, vomiting, diarrhea and constipation.
- Gavaj treatment, I/V therapy, oral fluid therapy.
- Urinary Catheters.
Nurse’s Responsibilities in Relation to Intravenous Drug Administration include:
- Knowing about the therapeutic use of medication or solution for administration, its normal amount, side effects, precautions, and contraindications.
- Prepare drugs aseptically and safely, inspect container and drug for defects, use correct diluents, and prepare immediately before administration.
- Be able to identify the patient and check for allergy status.
- Check prescription chart
- Check and maintain patency of vascular access devices.
- Inspect the site of vascular access devices and manage/report complications where appropriate.
- Control the flow rate of infusion and/or speed of injection.
- Monitor the patient’s condition and report changes.
- Make clear and immediate records of all drugs administered.
Nurses Guide to Intravenous Solutions

Common Intravenous therapy solutions:
Carbohydrates in Water (E.X.: 5% Dextrose in Water)
Possible Benefits From Administration Intravenous Therapy:
- Prevents dehydration.
- Prevents and treats ketosis.
- Promotes sodium diuresis (especially due to excessive administration of electrolyte solutions)
- Supplies calories (for energy)
- Supplies water (for body needs)
Conditions Requiring Precautions:
- Water intoxication
- Patients who are undergoing blood transfusions.
- Patients who are undergoing neurosurgical procedures.
Common Intravenous therapy solutions:
Carbohydrate in sodium chloride solution (Ex: 5% dextrose in half strength saline)
Possible Benefits From Administration Intravenous Therapy:
- Promotes diuresis.
- Prevents excessive fluid loss Corrects (due to perspiration).
- Prevents alkalosis.
- Provides calories and sodium chloride.
Conditions Requiring Precautions:
- Renal Insufficiency,
- Edema due to cardiac, hepatic and renal diseases.
Common Intravenous therapy solutions:
Sodium chloride solution (EX : 0.9 % sodium chloride solution)
Possible Benefits From Administration Intravenous Therapy:
- To treat alkalosis.
- To correct excessive fluid loss.
- To treat diabetic acidosis.
- To treat adrenal insufficiency.
- To treat vomiting from pyloric stenosis
Conditions Requiring Precautions:
- High sodium and plasma concentration.
- Dehydration.
- Hyponatremia.
Common Intravenous therapy solutions:
Ringer’s solution (sodium, chloride, potassium, and calcium)
Possible Benefits From Administration Intravenous Therapy:
- To treat dehydration due to reduced water intake.
- Vomiting.
- Diarrhea.
- To treat mild alkalosis.
- To treat hypochloremia.
Conditions Requiring Precautions:
- Additional Disease.
- Severe Potassium and Calcium Deficiency.
Common Intravenous Therapy Solution (Common Intravenous Therapy Solution) Intravenous therapy solutions):
Ringer lactate solution (content: sodium, potassium, calcium, chloride and lactate)
Possible Benefits From Administration Intravenous Therapy:
- To treat dehydration.
To restore normal fluid when extracellular fluid is lost For. - Moderate metabolic acidosis (renal insufficiency, infant diarrhea, diabetic acidosis)
- To replace daily extracellular electrolyte and water loss.
Conditions Requiring Precautions:
- Hepatic disease,
- Anoxia,
- Severe metabolic acidosis,
- Severe metabolic alkalosis.
Common Intravenous therapy solution solutions):
Multiple electrolyte solution (containing sodium, potassium, magnesium, chloride, lactate phosphate)
Possible Benefits From Administration Intravenous Therapy:
- To temporarily increase blood volume lost due to trauma, hemorrhage, burns, surgery, and anesthesia.
Conditions Requiring Precautions:
- Dextran allergy.