BSC SEM 1 UNIT 8 APPLIED BIOCHEMISTRY
UNIT 8 Immunochemistry
What is Immunochemistry?
Definition:
Immunochemistry is the branch of biochemistry that studies the chemical properties of immune reactions, particularly antigen-antibody interactions at a molecular level. It combines principles from immunology and biochemistry to explore and utilize the interactions between antigens and antibodies for diagnostic, therapeutic, and research purposes.
Key Components in Immunochemistry:
1. Antigens (Ag):
- Definition: Substances (proteins, polysaccharides, lipids, nucleic acids) that provoke an immune response.
- Types:
- Exogenous (foreign antigens)
- Endogenous (from body’s own tissues, sometimes auto-antigens)
- Epitope: Specific part of antigen recognized by antibodies.
2. Antibodies (Immunoglobulins, Ig):
- Structure: Y-shaped proteins produced by plasma cells (activated B cells).
- Structure:
- Two heavy chains and two light chains.
- Variable region (Fab region) binds specifically to antigen.
- Constant region (Fc region) mediates effector functions.
- Types: IgG, IgM, IgA, IgE, IgD.
2. Antigen-Antibody Interaction:
- Highly specific binding occurs through non-covalent interactions:
- Hydrogen bonds, electrostatic interactions, hydrophobic interactions, Van der Waals forces.
- Affinity: Strength of interaction between a single antigenic determinant and a single antibody binding site.
- Avidity: Overall binding strength between antibody and antigen (multiple binding sites).
Biochemical Principles in Immunochemistry:
1. Specificity:
- Antibody-antigen interactions are highly specific, relying on complementary shapes and biochemical interactions (hydrogen bonds, electrostatic interactions, hydrophobic interactions).
2. Affinity and Kinetics:
- Affinity: Measured by the equilibrium dissociation constant (Kd).
- Strong affinity: Leads to stronger and longer-lasting immune responses.
2. Non-Covalent Interactions:
- Hydrogen bonds, ionic interactions, Van der Waals forces, and hydrophobic interactions facilitate antibody-antigen binding.
3. Epitope (Antigenic Determinant):
- The precise region on the antigen recognized by antibodies.
Methods and Techniques in Immunochemistry:
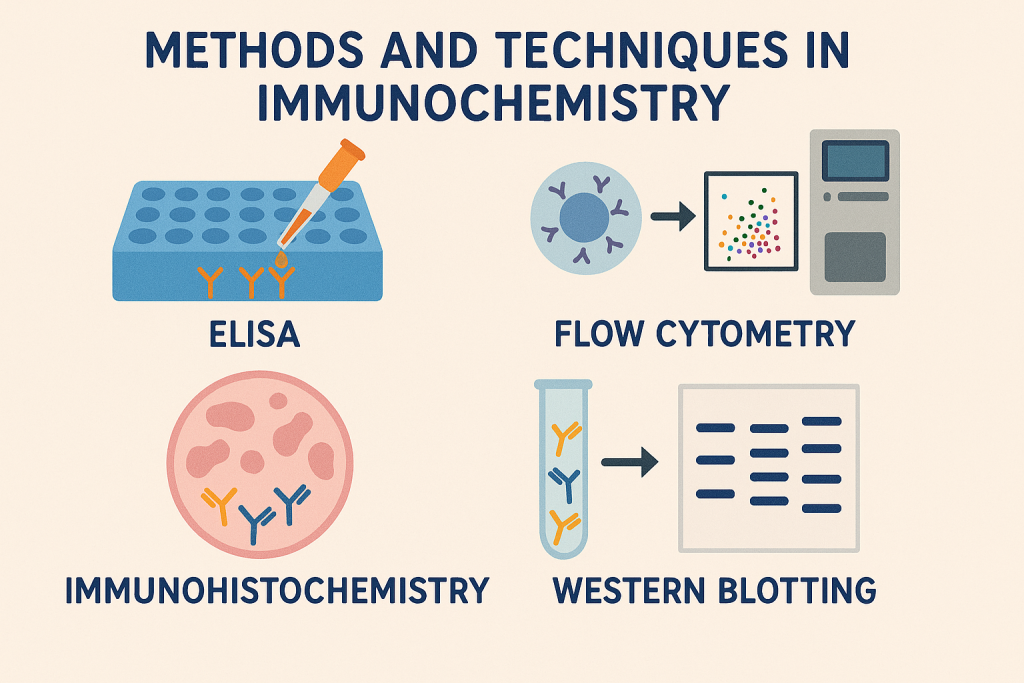
1. Immunoassays:
- ELISA (Enzyme-linked Immunosorbent Assay):
- Detects antigens or antibodies using enzyme-labeled antibodies.
- Used for disease diagnosis, hormone measurement, drug screening, etc.
2. Immunofluorescence (IF):
- Antibodies tagged with fluorescent molecules detect antigens in tissues or cells under a fluorescence microscope.
- Direct IF: Fluorophore-labeled antibody binds directly to antigen.
- Indirect IF: Secondary antibody labeled with fluorophore detects primary antibody-antigen complex.
4. Immunohistochemistry (IHC):
- Uses antibodies tagged with enzymes or dyes to visualize antigens in tissue sections microscopically.
5. Enzyme-linked Immunosorbent Assay (ELISA):
- Quantitative assay using antibodies linked with enzymes to measure antigens or antibodies in biological samples.
- Commonly used enzymes: Horseradish Peroxidase (HRP), Alkaline Phosphatase (ALP).
4. Western Blotting:
- Technique for detecting specific proteins using antibodies.
- Steps include:
- Protein separation by electrophoresis (SDS-PAGE).
- Transfer proteins onto membranes.
- Incubate membrane with specific antibodies for detection.
5. Immunohistochemistry (IHC):
- Localization of antigens within tissue sections using enzyme-linked antibodies.
- Visualization by enzyme-substrate reactions producing colored precipitates.
5. Enzyme-Linked Immunosorbent Assay (ELISA):
- Quantitative immunoassay for antigen or antibody detection.
- Types:
- Direct ELISA
- Indirect ELISA
- Sandwich ELISA
- Competitive ELISA
Clinical and Diagnostic Applications:
- Diagnosing infectious diseases (HIV, Hepatitis).
- Allergy testing (IgE detection).
- Cancer markers identification.
- Autoimmune disorders detection (e.g., rheumatoid arthritis, systemic lupus erythematosus).
Immunochemical Reagents:
- Primary antibodies: Bind directly to antigen.
- Secondary antibodies: Bind to primary antibodies; conjugated with detection molecules (enzymes, fluorophores).
- Conjugates: Antibodies linked chemically to enzymes or fluorescent dyes for visualization.
Biochemical Importance in Nursing and Medical Field:
- Disease Diagnosis: Immunochemistry techniques (ELISA, IF, western blotting) used in diagnosis of infectious diseases, autoimmune disorders, and cancer biomarkers.
- Therapeutic Monitoring: Measuring drug concentrations, hormones, tumor markers.
- Research Applications: Studying pathogenesis, drug discovery, and vaccine development.
Clinical Applications:
- Immunohistochemistry: Identification of tumor markers (e.g., HER2, ER, PR in breast cancer).
- Western Blotting: Confirmatory test in HIV diagnosis.
- ELISA: Screening tests for infectious diseases (HIV, Hepatitis).
Applications in Nursing and Health Sciences:
- Understanding disease pathology and management.
- Drug monitoring (therapeutic drug monitoring).
- Vaccine evaluation and immunogenicity testing.
- Research on immune system disorders.
Future Perspectives in Immunochemistry:

- Advanced biosensor technologies.
- Development of monoclonal antibodies and targeted immunotherapies.
- Personalized medicine based on immunochemical markers.
Immunochemistry: Structure, Functions, and Biochemistry of Immunoglobulins (Antibodies)
What are Immunoglobulins?
Immunoglobulins (Ig), commonly known as antibodies, are specialized glycoproteins produced by plasma cells (activated B-lymphocytes). They play a critical role in the immune response by recognizing and neutralizing pathogens such as bacteria and viruses.
Biochemical Structure of Immunoglobulins
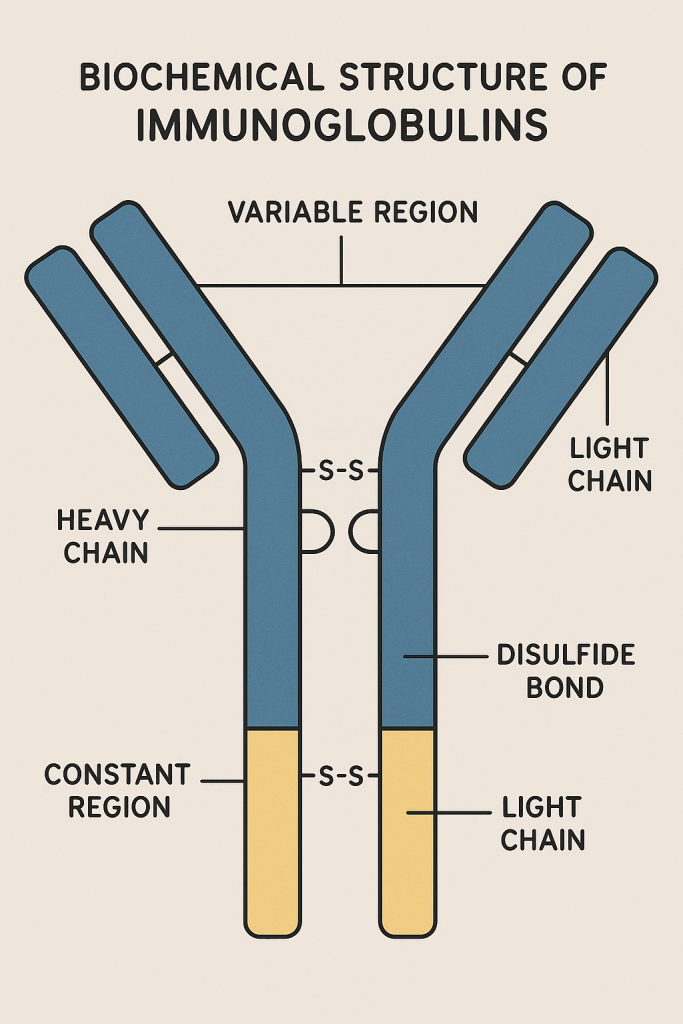
Each immunoglobulin molecule is composed of four polypeptide chains:
- Two Heavy (H) chains: (~50-75 kDa each)
- Two Light chains: (Kappa κ or Lambda λ; about 25 kDa each)
The heavy and light chains are linked by disulfide bonds.
Basic Structure:
- Y-shaped molecule:
- Two identical antigen-binding sites (Fab regions).
- A constant region (Fc region) responsible for biological functions.
Domains:
- Variable (V) region: Contains antigen-binding sites; varies between antibodies.
- Constant region (C region): Responsible for mediating biological functions.
Heavy Chains (5 types/classes):
Each immunoglobulin type is named according to the heavy chain type:
| Immunoglobulin | Heavy Chain | Subtypes |
|---|---|---|
| IgG | Gamma (γ) | γ1, γ2, γ3, γ4 |
| IgM | Mu (μ) | – |
| IgA | Alpha (α) | α1, α2 |
| IgD | Delta (δ) | – |
| IgE | Epsilon (ε) | – |
Classes and Functions of Immunoglobulins:
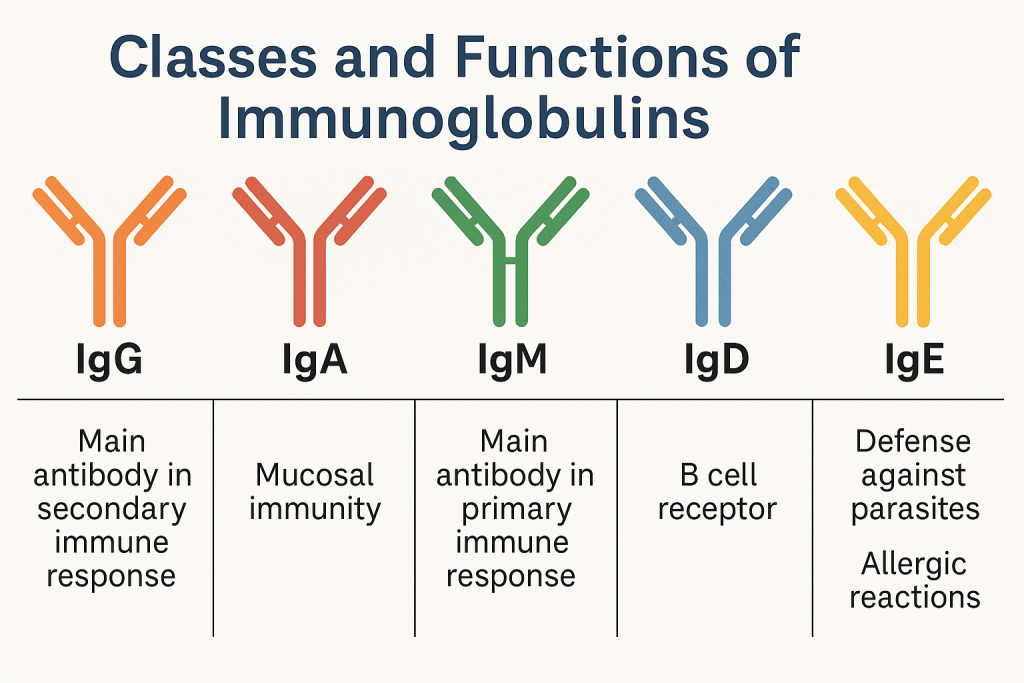
1. IgG (Gamma globulin)
- Structure: Monomer (single Y-shaped unit)
- Concentration: Most abundant antibody in plasma (~75% of serum antibodies).
- Function:
- Main antibody in secondary immune response.
- Opsonization (enhances phagocytosis).
- Neutralization of toxins and viruses.
- Crosses placenta providing passive immunity to the fetus.
- Activates complement system.
2. IgM (Macroglobulin)
- Structure: Pentamer (5 Y-shaped units linked by J-chain)
- Concentration: ~10% of serum antibodies.
- Function:
- Primary immune response antibody.
- First antibody produced during infection.
- Strongly activates complement system.
- Indicator of recent infection.
3. IgA (Secretory antibody)
- Structure:
- Monomer in serum, Dimer in secretions (tears, saliva, breast milk, mucus).
- Secretory component for mucosal protection.
- Function:
- Protects mucosal surfaces (GI tract, respiratory tract, urogenital tract).
- Provides immunity in secretions (breast milk, saliva, tears).
- Prevents attachment of pathogens to mucosal surfaces.
4. IgE (Reaginic antibody)
- Structure: Monomer
- Function:
- Important in allergic reactions and parasitic infections.
- Binds strongly to mast cells and basophils.
- Releases histamine upon antigen binding, causing inflammatory response (e.g., asthma, anaphylaxis).
5. IgD
- Structure: Monomer, low concentration in plasma
- Function:
- Acts primarily as an antigen receptor on naive B cells.
- Little known about specific functions.
- Possibly involved in B-cell differentiation.
Biochemical Features of Immunoglobulins:
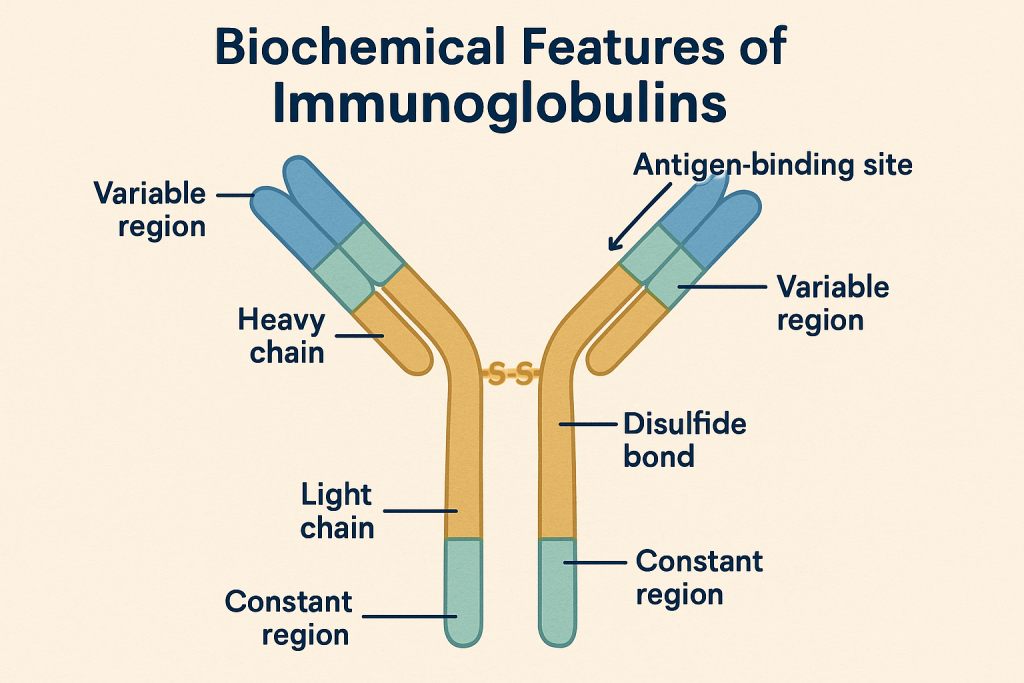
Glycoprotein nature:
- Composed of proteins with carbohydrate side chains (glycosylation).
- Glycosylation stabilizes antibodies and modulates effector functions.
Disulfide bonds:
- Provide structural stability and flexibility.
- Essential for antigen-binding specificity and affinity.
Antigen Binding:
- Highly specific due to complementary shape and charges.
- Non-covalent bonds: hydrogen bonding, ionic bonds, Van der Waals forces, hydrophobic interactions.
Variable and Constant Regions:
- Variable region (V-region): Unique amino acid sequences determine specificity for antigen binding.
- Constant region (C-region): Determines antibody class and effector functions (complement binding, binding to immune cells).
Clinical and Diagnostic Applications of Immunoglobulins:
Diagnostic uses:
- Serological tests: ELISA, Western blotting, Immunofluorescence assays.
- Identification of infections: Detection of specific antibodies (IgG, IgM) helps diagnose infections.
- Autoimmune disorders: Detection of autoantibodies.
Laboratory Techniques in Immunochemistry involving Immunoglobulins:
- Immunofluorescence
- Western Blotting
- ELISA (Enzyme-Linked Immunosorbent Assay)
- Immunohistochemistry
- Flow cytometry
Summary Table of Immunoglobulin Classes:
| Feature | IgG | IgM | IgA | IgE | IgD |
|---|---|---|---|---|---|
| Structure | Monomer | Pentamer | Dimer/Monomer | Monomer | Monomer |
| Molecular Weight | 150 kDa | 900 kDa | 320 kDa | 200 kDa | 180 kDa |
| Half-life | 21 days | 5 days | 6 days | 2 days | 2-3 days |
| Serum Conc. | Highest (~75%) | ~10% | ~10-15% | Trace amounts | <1% |
Enzyme-Linked Immunosorbent Assay (ELISA)
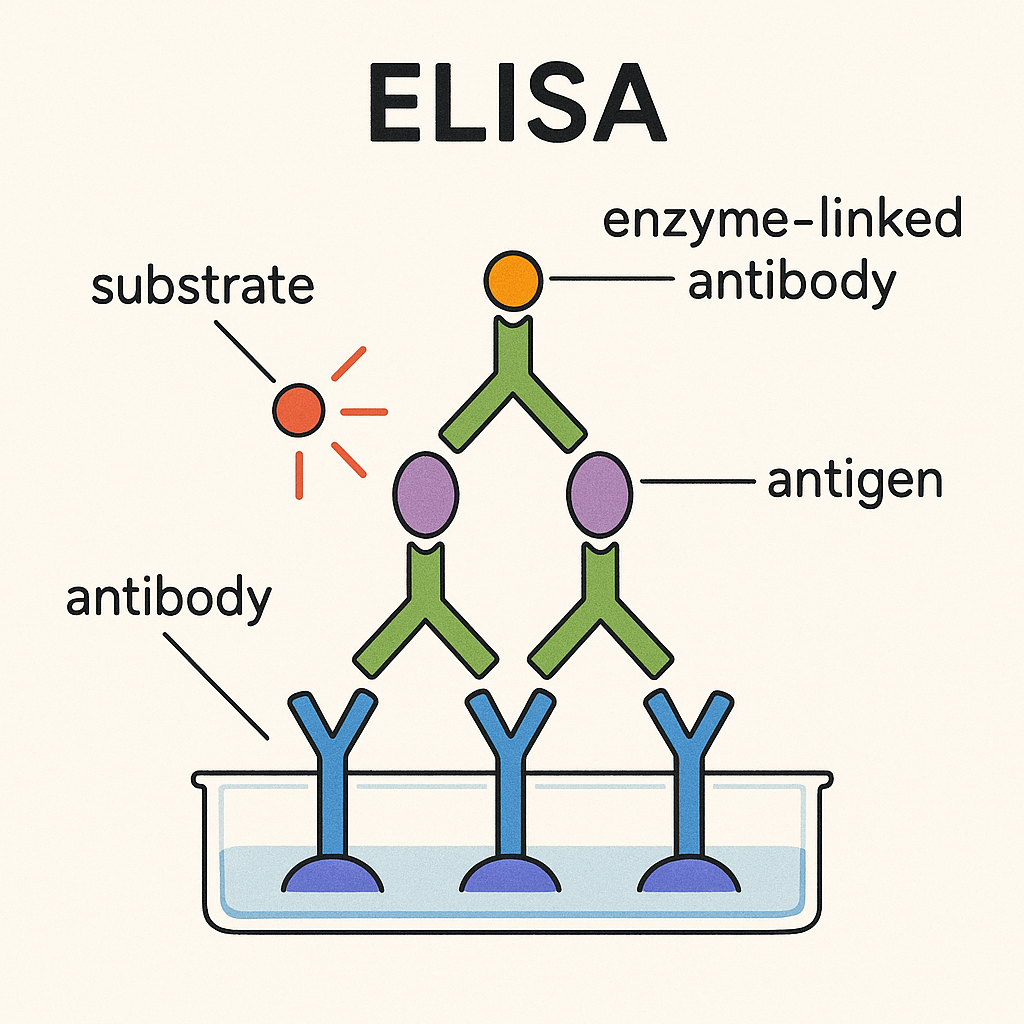
Introduction
- ELISA is a biochemical technique used widely in immunochemistry to detect and quantify antigens or antibodies in a sample.
- It combines the specificity of antibodies with the catalytic efficiency of enzymes for sensitive detection.
Basic Principle of ELISA
ELISA is based on antigen-antibody interactions and enzyme-substrate reactions:
- Specific antibodies bind to their respective antigens.
- Enzyme-labeled antibodies produce color reactions that can be quantitatively measured.
Key Components in ELISA
- Antigen: Target molecule of detection (protein, hormone, pathogen).
- Antibody: Binds specifically to antigens; can be primary or secondary.
- Enzyme conjugate: Antibody linked to enzyme (e.g., Horseradish peroxidase [HRP], Alkaline phosphatase [ALP]).
- Substrate (chromogen): Chemical that reacts with enzyme to produce color.
- Common substrates: TMB (HRP), PNPP (ALP substrate).
Types of ELISA:
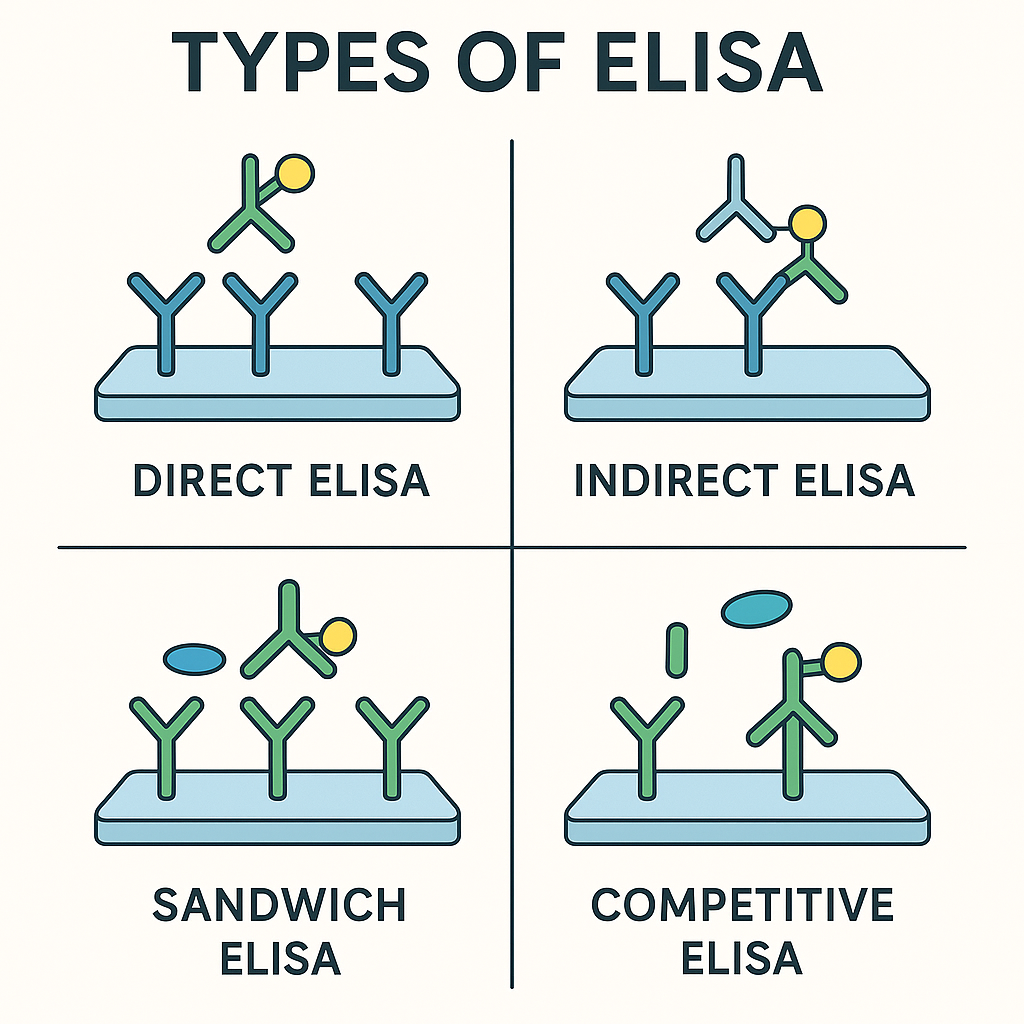
| Type | Detection Target | Sensitivity | Key Features | |————————|——————|—————————-| | Direct ELISA | Antigen | Simple, rapid but less sensitive | | Indirect ELISA | Antibody | Highly sensitive; common for screening | | Sandwich ELISA | Antigen | Highest specificity and sensitivity | | Competitive ELISA | Antigen/Antibody | Ideal for small molecules; highly sensitive |
Detailed Steps in ELISA Technique
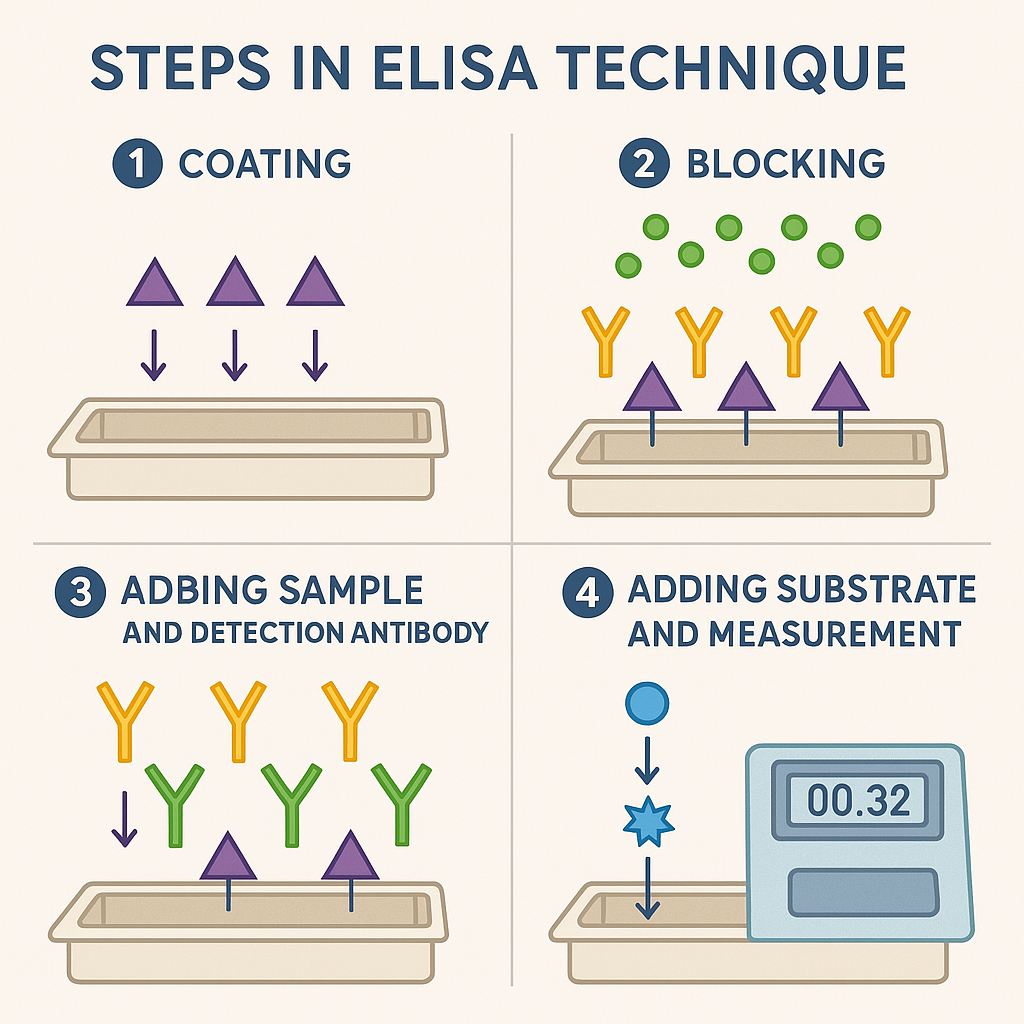
ELISA is typically performed in microtiter plates (96 wells):
- Coating:
- Antigen or antibody immobilized onto microplate surface.
- Blocking:
- Unoccupied binding sites are blocked with proteins (e.g., bovine serum albumin [BSA]) to prevent nonspecific binding.
- Sample Addition:
- Add the biological sample (e.g., serum, plasma, urine) containing antigen or antibody.
- Addition of Detection Antibody:
- Enzyme-conjugated antibody binds specifically to the antigen-antibody complex.
- Washing Steps:
- Wash away unbound materials to ensure specificity.
- Addition of Substrate:
- Enzyme reacts with substrate, producing a measurable signal (colorimetric or fluorescent).
- Measurement:
- Quantify the reaction product (color intensity) using spectrophotometry.
Enzymes commonly used in ELISA
| Enzyme | Substrate | Product Appearance |
|---|---|---|
| Horseradish peroxidase (HRP) | TMB (Tetramethylbenzidine), OPD | Blue (turns yellow after stopping reaction) |
| Alkaline phosphatase (ALP) | p-Nitrophenyl phosphate (pNPP) | Yellow |
Applications of ELISA in Biochemistry & Clinical Practice

1. Diagnostic Applications
- Infectious diseases: Detection of antibodies or antigens (e.g., HIV, Hepatitis, COVID-19).
- Hormone assays: Measurement of hormones like TSH, LH, Insulin, HCG.
- Autoimmune disorders: Detection of autoantibodies (e.g., rheumatoid factor, ANA).
- Allergy testing: Quantification of IgE antibodies specific to allergens.
2. Research Applications
- Quantitative protein analysis.
- Cytokine detection in inflammation research.
- Analysis of biomarkers for research and drug development.
3. Pharmaceutical Industry
- Testing drug efficacy by measuring specific biomarkers.
- Monitoring therapeutic antibody levels in patients.
Advantages of ELISA
- Highly specific and sensitive.
- Quantitative analysis possible.
- Can be automated for high-throughput analysis.
- Versatile application for many biological analytes.
Limitations of ELISA
- Potential for cross-reactivity or nonspecific binding.
- False-positive/negative results possible.
- Requires quality antibodies and careful standardization.
Troubleshooting Common Problems in ELISA
| Problem | Possible Reason | Solution |
|---|---|---|
| High background | Non-specific binding | Improve blocking step |
| Weak signals | Low antigen/antibody concentration | Increase sample concentration |
| No color development | Incorrect enzyme/substrate pairing | Ensure enzyme-substrate compatibility |
| Variable results | Pipetting error, incubation variability | Standardize procedure & calibrate equipment |
Biochemical Considerations in ELISA
- Specificity: Antigen-antibody interactions due to complementary molecular shapes and chemical interactions.
- Affinity and avidity: Determine effectiveness of antigen-antibody binding.
- Enzyme activity: Influenced by optimal pH, temperature, incubation time.
Applications in Nursing and Health Sciences
- Early disease detection and patient monitoring.
- Hormonal imbalances and reproductive health testing.
- Community-level epidemiological surveillance.