BSC SEM 1 UNIT 5 APPLIED BIOCHEMISTRY
UNIT 5 Acid base maintenance.
Acid-Base Maintenance:
Introduction
Acid-base balance refers to the regulation of hydrogen ion (H⁺) concentration in the body to maintain the normal pH of blood and tissues. The body’s pH is maintained between 7.35 and 7.45, which is essential for enzymatic functions, cellular activities, and overall homeostasis.
1. pH and Its Significance in Biochemistry
- pH Definition: The pH is the negative logarithm of the hydrogen ion concentration: pH=−log[H+]pH = -\log[H^+]pH=−log[H+]
- Normal blood pH: 7.35–7.45
- Acidosis: pH < 7.35 (excess H⁺)
- Alkalosis: pH > 7.45 (excess OH⁻)
- Biochemical Relevance:
- Affects enzyme activity, protein stability, and membrane potential.
- Important in metabolic reactions, especially in cellular respiration.
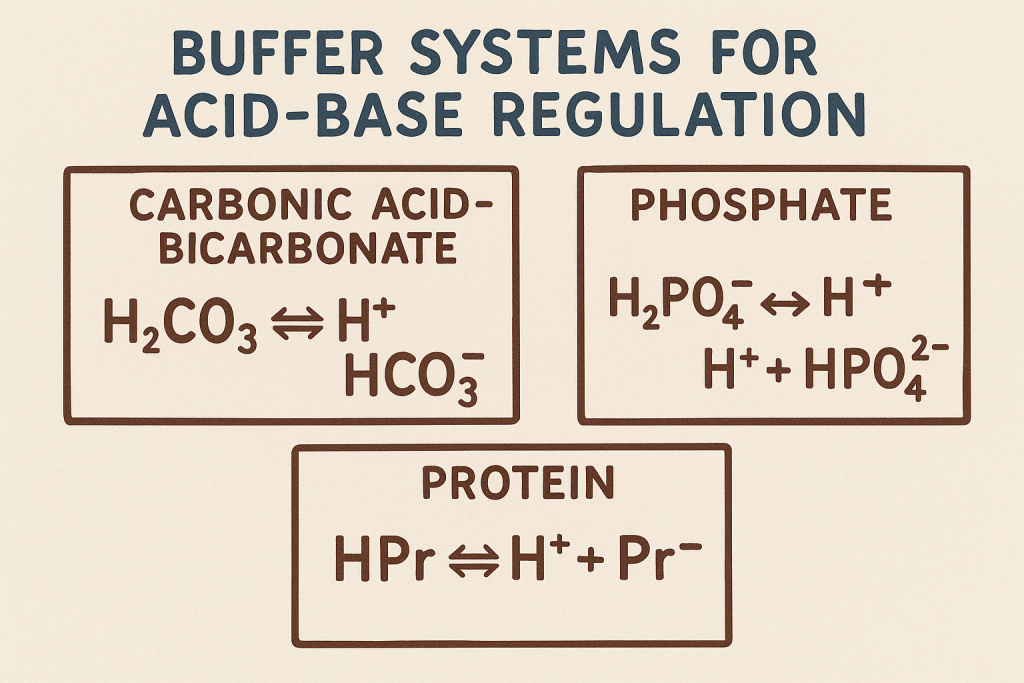
2. Buffer Systems for Acid-Base Regulation
The body uses buffer systems to maintain pH balance:
A. Bicarbonate (HCO₃⁻) Buffer System (Primary Extracellular Buffer)
Equation: H2CO3⇌H++HCO3−H_2CO_3 \rightleftharpoons H^+ + HCO_3^-H2CO3⇌H++HCO3− CO2+H2O⇌H2CO3⇌H++HCO3−CO_2 + H_2O \rightleftharpoons H_2CO_3 \rightleftharpoons H^+ + HCO_3^-CO2+H2O⇌H2CO3⇌H++HCO3−
- Maintains pH by balancing carbonic acid (H₂CO₃) and bicarbonate (HCO₃⁻).
- Controlled by the lungs (CO₂ regulation) and kidneys (HCO₃⁻ reabsorption/excretion).
B. Phosphate Buffer System (Important in Renal Tubules)
Equation: H2PO4−⇌H++HPO42−H_2PO_4^- \rightleftharpoons H^+ + HPO_4^{2-}H2PO4−⇌H++HPO42−
- Helps maintain pH in intracellular fluids and urine.
- Plays a key role in kidney function.
C. Protein Buffer System (Intracellular and Plasma Proteins)
- Proteins like hemoglobin and albumin act as buffers.
- Histidine residues in proteins accept or donate H⁺ to stabilize pH.
D. Hemoglobin Buffer System (Oxygen Transport and CO₂ Regulation)
- In RBCs, hemoglobin binds H⁺ and CO₂ to regulate pH.
- Facilitates Bohr effect, allowing oxygen release in acidic tissues.
3. Role of Organs in Acid-Base Balance
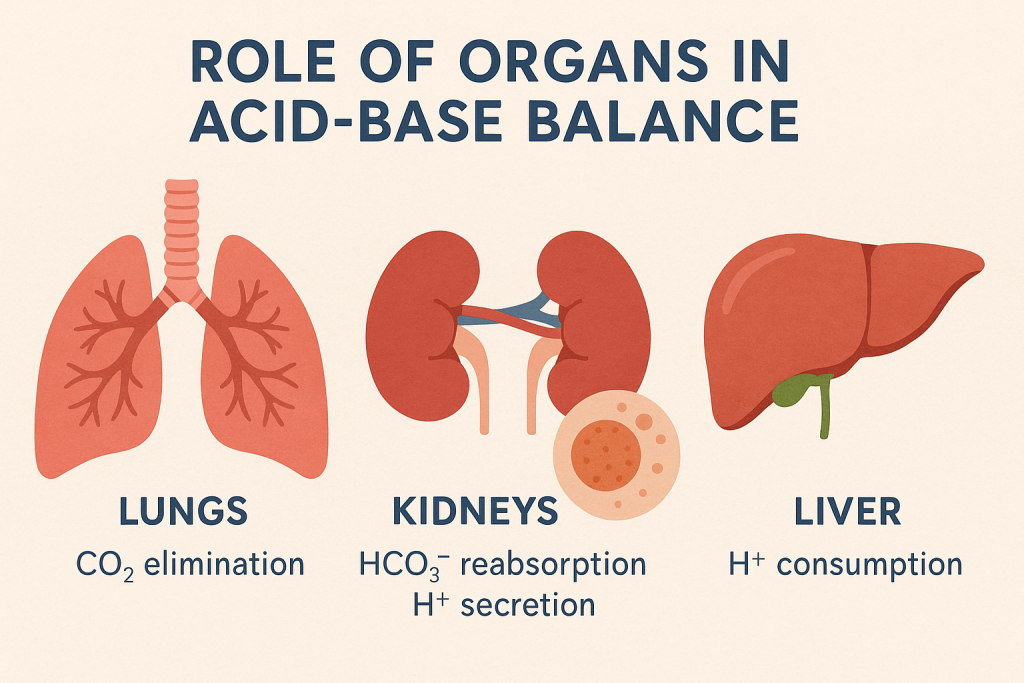
A. Lungs (Respiratory Regulation)
- Control CO₂ levels via breathing.
- Hyperventilation → Decreases CO₂ → Increases pH (alkalosis).
- Hypoventilation → Increases CO₂ → Decreases pH (acidosis).
B. Kidneys (Metabolic Regulation)
- Regulate HCO₃⁻ reabsorption and H⁺ excretion.
- Renal Tubular Mechanism:
- Proximal tubule: Reabsorbs HCO₃⁻.
- Distal tubule: Secretes H⁺ into urine.
C. Liver
- Produces urea cycle intermediates, balancing NH₄⁺ and acid excretion.
- Important in protein metabolism and acid-base homeostasis.
4. Acid-Base Disorders (Biochemical Perspective)
| Disorder | Causes | Biochemical Markers |
|---|---|---|
| Respiratory Acidosis | Hypoventilation (CO₂ retention) | ↑ CO₂, ↓ pH, ↑ HCO₃⁻ compensation |
| Respiratory Alkalosis | Hyperventilation (CO₂ loss) | ↓ CO₂, ↑ pH, ↓ HCO₃⁻ compensation |
| Metabolic Acidosis | Excess acid (lactic acidosis, ketoacidosis) | ↓ HCO₃⁻, ↓ pH, compensatory hyperventilation |
| Metabolic Alkalosis | Excess base (vomiting, diuretics) | ↑ HCO₃⁻, ↑ pH, compensatory hypoventilation |
5. Biochemical Tests for Acid-Base Balance
- Arterial Blood Gas (ABG) Analysis:
- pH, pCO₂, HCO₃⁻ levels.
- Anion Gap Calculation: Anion Gap=(Na++K+)−(Cl−+HCO3−)\text{Anion Gap} = (\text{Na}^+ + \text{K}^+) – (\text{Cl}^- + \text{HCO}_3^-)Anion Gap=(Na++K+)−(Cl−+HCO3−)
- Normal: 8–12 mEq/L
- High Anion Gap → Metabolic acidosis.
- Base Excess:
- Indicates metabolic contribution to pH imbalance.
6. Acid-Base Imbalance and Nursing Implications
- Monitor ABG reports regularly.
- Administer oxygen therapy for respiratory imbalances.
- Correct fluid-electrolyte balance in metabolic disorders.
- Educate patients on breathing exercises for respiratory alkalosis.
- Administer IV bicarbonate for severe metabolic acidosis.
pH: Definition, Normal Values,
Definition of pH
pH is a measure of the hydrogen ion concentration (H⁺) in a solution, indicating its acidity or alkalinity. It is mathematically expressed as: pH=−log[H+]pH = -\log[H^+]pH=−log[H+]
This equation means that as the hydrogen ion concentration increases, the pH decreases, making the solution more acidic. Conversely, a decrease in hydrogen ion concentration increases pH, making the solution more alkaline (basic).
Normal pH Values in the Body
- Arterial blood pH: 7.35 – 7.45 (Slightly alkaline)
- Venous blood pH: 7.31 – 7.41 (Slightly lower due to CO₂ accumulation)
- Intracellular pH: Varies between 6.8 – 7.4, depending on the cell type and function.
- Urine pH: Normally between 4.5 – 8.0 (varies depending on diet and hydration).
- Gastric juice pH: 1.5 – 3.5 (Highly acidic for digestion).
- Pancreatic juice pH: 8.0 – 8.3 (Highly alkaline to neutralize gastric acid in the intestine).
- CSF (Cerebrospinal fluid) pH: 7.3 – 7.4.
pH Scale and Its Significance
- The pH scale ranges from 0 to 14.
- pH < 7.0 → Acidic (Higher H⁺ concentration)
- pH = 7.0 → Neutral
- pH > 7.0 → Alkaline (Basic) (Lower H⁺ concentration)
| pH Level | Examples |
|---|---|
| 0 – 1 | Strong acids (Battery acid, HCl) |
| 2 – 3 | Gastric acid, Lemon juice |
| 4 – 5 | Acidic foods (Tomato juice, Wine) |
| 6 – 7 | Milk, Pure water |
| 8 – 9 | Seawater, Baking soda |
| 10 – 11 | Milk of magnesia, Mild detergents |
| 12 – 13 | Soapy water, Bleach |
| 14 | Strong bases (NaOH – Sodium Hydroxide) |
Role of pH in Biochemical Reactions
1. Enzyme Activity
- Enzymes function optimally at specific pH levels.
- Example:
- Pepsin (Stomach enzyme): Active at pH 1.5 – 2.0.
- Trypsin (Intestinal enzyme): Active at pH 8.0 – 8.5.
- Amylase (Salivary enzyme): Works best at pH 6.7 – 7.0.
2. Protein Structure
- pH influences protein folding and function.
- Extreme pH changes can cause denaturation, rendering proteins inactive.
3. Membrane Potential & Nerve Function
- Hydrogen ions (H⁺) affect ion channels, altering nerve impulse transmission.
- pH changes can disrupt action potentials in neurons and muscles.
4. Hemoglobin Function
- Bohr Effect: At low pH (acidic conditions), hemoglobin releases more oxygen to tissues.
- At high pH (alkaline conditions), hemoglobin binds oxygen more tightly.
5. Metabolic Pathways
- Cellular metabolism generates acidic (H⁺) and basic (HCO₃⁻) products.
- Glycolysis, Krebs cycle, and oxidative phosphorylation influence pH.
Buffer Systems That Maintain pH
To maintain a normal pH (7.35 – 7.45), the body has several buffer systems:
1. Bicarbonate (HCO₃⁻) Buffer System
CO2+H2O⇌H2CO3⇌HCO3−+H+CO_2 + H_2O \rightleftharpoons H_2CO_3 \rightleftharpoons HCO_3^- + H^+CO2+H2O⇌H2CO3⇌HCO3−+H+
- Regulates blood pH by balancing carbonic acid (H₂CO₃) and bicarbonate (HCO₃⁻).
- Lungs and kidneys regulate this buffer system.
2. Phosphate Buffer System
H2PO4−⇌H++HPO42−H_2PO_4^- \rightleftharpoons H^+ + HPO_4^{2-}H2PO4−⇌H++HPO42−
- Functions inside cells and in the kidneys.
- Important for urine pH regulation.
3. Protein Buffer System
- Hemoglobin, Albumin, and Amino Acids help maintain pH.
- Example: Hemoglobin binds H⁺ and CO₂ to regulate blood pH.
4. Respiratory System (CO₂ Regulation)
- Lungs control CO₂ levels, influencing pH.
- Hyperventilation → ↓ CO₂ → ↑ pH (Respiratory Alkalosis).
- Hypoventilation → ↑ CO₂ → ↓ pH (Respiratory Acidosis).
5. Renal System (H⁺ & HCO₃⁻ Regulation)
- Kidneys regulate pH by excreting H⁺ ions and reabsorbing HCO₃⁻.
- Slow but powerful system for long-term acid-base balance.
Acid-Base Disorders and Their Biochemical Causes
| Condition | pH | Cause | Compensation |
|---|---|---|---|
| Respiratory Acidosis | ↓ pH (<7.35) | Hypoventilation (↑ CO₂ retention) | Kidneys retain HCO₃⁻ |
| Respiratory Alkalosis | ↑ pH (>7.45) | Hyperventilation (↓ CO₂) | Kidneys excrete HCO₃⁻ |
| Metabolic Acidosis | ↓ pH (<7.35) | ↑ Acid production (lactic acidosis, ketoacidosis) or ↓ HCO₃⁻ | Lungs increase ventilation (↓ CO₂) |
| Metabolic Alkalosis | ↑ pH (>7.45) | Loss of acids (vomiting, diuretics) or ↑ HCO₃⁻ intake | Lungs decrease ventilation (↑ CO₂) |
Laboratory Tests for pH and Acid-Base Balance
- Arterial Blood Gas (ABG) Analysis:
- Measures pH, pCO₂, HCO₃⁻ levels.
- Normal values:
- pH: 7.35 – 7.45
- pCO₂: 35 – 45 mmHg
- HCO₃⁻: 22 – 26 mEq/L
- Serum Electrolytes:
- Na⁺, K⁺, Cl⁻, HCO₃⁻ help assess pH imbalances.
- Anion Gap Calculation: Anion Gap=(Na++K+)−(Cl−+HCO3−)\text{Anion Gap} = (\text{Na}^+ + \text{K}^+) – (\text{Cl}^- + \text{HCO}_3^-)Anion Gap=(Na++K+)−(Cl−+HCO3−)
- High Anion Gap → Metabolic Acidosis.
Nursing and Medical Interventions for pH Imbalances
- Monitor ABG reports and correct imbalances.
- Respiratory Acidosis:
- Administer oxygen, improve ventilation.
- Respiratory Alkalosis:
- Encourage slow breathing (breathing into a paper bag).
- Metabolic Acidosis:
- Administer IV bicarbonate if severe.
- Correct underlying cause (e.g., diabetes, renal failure).
- Metabolic Alkalosis:
- Hydration with NaCl solutions, correct electrolyte imbalance.
Regulation of Blood pH
Introduction
The regulation of blood pH is essential for maintaining homeostasis and ensuring normal physiological functions. The normal blood pH is maintained between 7.35 – 7.45. If the pH falls below or rises above this range, it can lead to acidosis (pH < 7.35) or alkalosis (pH > 7.45), which can be life-threatening.
The body regulates blood pH through buffer systems, respiratory control, and renal mechanisms.
Mechanisms of Blood pH Regulation
The three major systems responsible for maintaining blood pH are:
1. Buffer Systems (Immediate Response)
Buffer systems act within seconds to resist pH changes by neutralizing excess acids or bases.
A. Bicarbonate (HCO₃⁻) Buffer System (Primary Buffer)
CO2+H2O⇌H2CO3⇌H++HCO3−CO_2 + H_2O \rightleftharpoons H_2CO_3 \rightleftharpoons H^+ + HCO_3^-CO2+H2O⇌H2CO3⇌H++HCO3−
- The most important extracellular buffer system.
- If blood pH drops (acidosis): HCO₃⁻ binds with H⁺ to form H₂CO₃, which dissociates into CO₂ and H₂O. The lungs then remove excess CO₂.
- If blood pH rises (alkalosis): H₂CO₃ dissociates into H⁺ and HCO₃⁻, releasing H⁺ to lower pH.
B. Phosphate Buffer System (Intracellular and Renal Buffer)
H2PO4−⇌H++HPO42−H_2PO_4^- \rightleftharpoons H^+ + HPO_4^{2-}H2PO4−⇌H++HPO42−
- Works in the intracellular fluid (ICF) and renal tubules.
- Helps regulate urine pH.
C. Protein Buffer System (Hemoglobin, Albumin, Amino Acids)
- Hemoglobin (Hb) Buffer:
- In RBCs, hemoglobin binds H⁺ and CO₂, preventing pH changes.
- In tissues, hemoglobin releases O₂ and binds CO₂ and H⁺.
- In lungs, hemoglobin releases CO₂ and H⁺, helping to maintain pH.
- Plasma Proteins (Albumin, Globulins)
- Proteins contain amino (-NH₂) and carboxyl (-COOH) groups, which can accept or release H⁺.
2. Respiratory System (Minutes)
The lungs play a crucial role in regulating blood pH by controlling carbon dioxide (CO₂) levels through breathing.
- Hypoventilation (Slow Breathing):
- Retains CO₂ → Increases H₂CO₃ → Lowers pH (Acidosis).
- Seen in COPD, pneumonia, drug overdose.
- Hyperventilation (Rapid Breathing):
- Expels CO₂ → Reduces H₂CO₃ → Raises pH (Alkalosis).
- Seen in anxiety, fever, hypoxia.
Respiratory Compensation
- If pH drops (acidosis), the respiratory rate increases to eliminate CO₂.
- If pH rises (alkalosis), the respiratory rate decreases to retain CO₂.
3. Renal System (Slow but Long-Term Regulation)
The kidneys regulate blood pH by:
- Reabsorbing Bicarbonate (HCO₃⁻):
- In acidosis, the kidneys reabsorb more HCO₃⁻ to neutralize acids.
- In alkalosis, the kidneys excrete excess HCO₃⁻ to lower pH.
- Excreting Hydrogen Ions (H⁺)
- In acidosis, the kidneys secrete more H⁺ into urine.
- H⁺ combines with phosphate (H₂PO₄⁻) or ammonia (NH₃) to form excretable acids.
- Producing New Bicarbonate (HCO₃⁻)
- The kidneys synthesize bicarbonate to balance blood pH.
Renal Compensation
- In acidosis: The kidneys increase H⁺ excretion and reabsorb more HCO₃⁻.
- In alkalosis: The kidneys excrete HCO₃⁻ and retain H⁺.
Acid-Base Disorders and Compensation
| Disorder | Primary Cause | pH Level | Compensation Mechanism |
|---|---|---|---|
| Respiratory Acidosis | Hypoventilation (↑ CO₂) | ↓ pH (<7.35) | Kidneys retain HCO₃⁻, excrete H⁺ |
| Respiratory Alkalosis | Hyperventilation (↓ CO₂) | ↑ pH (>7.45) | Kidneys excrete HCO₃⁻, retain H⁺ |
| Metabolic Acidosis | ↑ Acid production (lactic acidosis, ketoacidosis) or ↓ HCO₃⁻ | ↓ pH (<7.35) | Lungs hyperventilate to remove CO₂ |
| Metabolic Alkalosis | Loss of acid (vomiting, diuretics) or ↑ HCO₃⁻ intake | ↑ pH (>7.45) | Lungs hypoventilate to retain CO₂ |
Laboratory Tests for pH Regulation
- Arterial Blood Gas (ABG) Test:
- pH: 7.35 – 7.45
- pCO₂: 35 – 45 mmHg (Respiratory component)
- HCO₃⁻: 22 – 26 mEq/L (Metabolic component)
- Serum Electrolytes:
- Na⁺, K⁺, Cl⁻, HCO₃⁻ help assess pH imbalances.
- Anion Gap Calculation:
- Normal: 8 – 12 mEq/L
- High anion gap acidosis → Lactic acidosis, ketoacidosis.
Nursing and Medical Interventions for pH Imbalance
A. Respiratory Acidosis (Low pH, High CO₂)
- Administer oxygen therapy.
- Improve ventilation (mechanical ventilation if needed).
- Treat underlying cause (e.g., COPD, pneumonia).
B. Respiratory Alkalosis (High pH, Low CO₂)
- Encourage slow breathing (paper bag method).
- Treat anxiety, pain, or fever.
- Monitor blood gases.
C. Metabolic Acidosis (Low pH, Low HCO₃⁻)
- Correct underlying cause (diabetic ketoacidosis, renal failure).
- Administer IV sodium bicarbonate (if severe).
- Hydration and electrolyte correction.
D. Metabolic Alkalosis (High pH, High HCO₃⁻)
- Administer IV fluids (normal saline, potassium chloride).
- Correct underlying cause (vomiting, diuretic overuse).
- Monitor urine output and blood gases.
Blood Buffer System: Regulation of pH in Blood
Introduction
The blood buffer system is a biochemical mechanism that helps maintain the pH of blood within the normal range (7.35 – 7.45). It prevents sudden changes in hydrogen ion (H⁺) concentration, which is crucial for enzyme function, metabolism, and cellular activities.
The body maintains acid-base balance using three major buffer systems in the blood:
- Bicarbonate (HCO₃⁻) Buffer System
- Phosphate (H₂PO₄⁻) Buffer System
- Protein Buffer System (Hemoglobin and Plasma Proteins)
1. Bicarbonate (HCO₃⁻) Buffer System (Primary Blood Buffer)
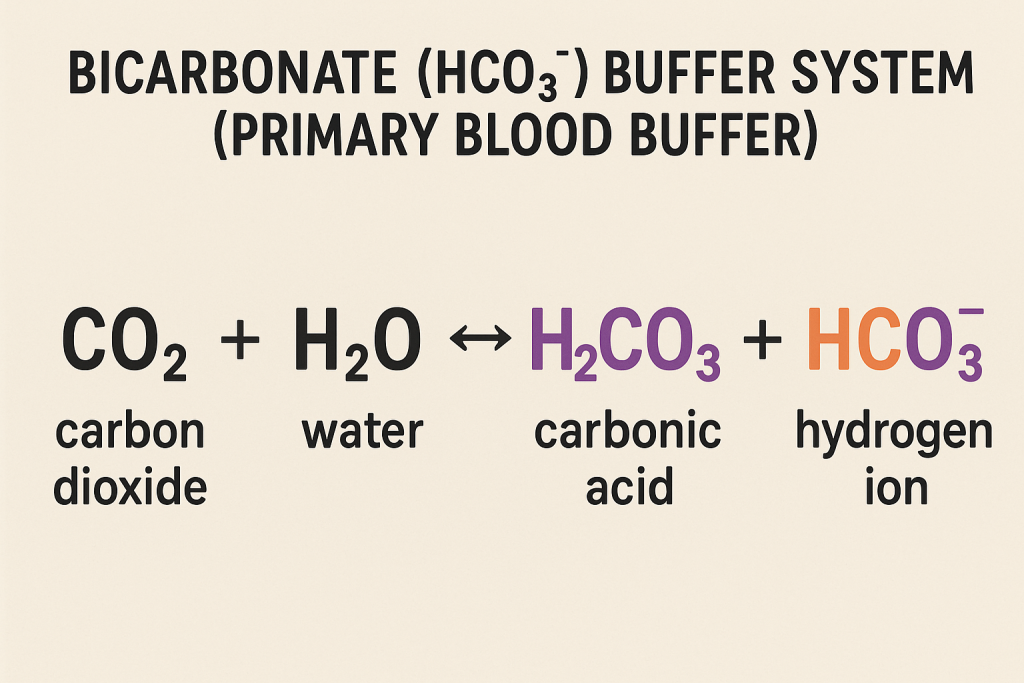
This is the most important extracellular buffer system, responsible for maintaining blood pH.
Reaction:
CO2+H2O⇌H2CO3⇌HCO3−+H+CO_2 + H_2O \rightleftharpoons H_2CO_3 \rightleftharpoons HCO_3^- + H^+CO2+H2O⇌H2CO3⇌HCO3−+H+
- When pH decreases (Acidosis, excess H⁺):
- HCO₃⁻ binds to H⁺ forming H₂CO₃ (carbonic acid).
- Carbonic acid dissociates into CO₂ and H₂O.
- CO₂ is expelled through lungs, increasing pH.
- When pH increases (Alkalosis, excess OH⁻):
- H₂CO₃ dissociates, releasing H⁺ to lower pH.
Regulation by Lungs and Kidneys
- Lungs: Regulate pH by exhaling CO₂ (faster breathing raises pH, slower breathing lowers pH).
- Kidneys: Regulate pH by reabsorbing HCO₃⁻ and excreting H⁺.
Clinical Significance
- Respiratory Acidosis: Hypoventilation (CO₂ retention, ↓ pH).
- Respiratory Alkalosis: Hyperventilation (CO₂ loss, ↑ pH).
- Metabolic Acidosis: Low HCO₃⁻ levels (e.g., ketoacidosis, lactic acidosis).
- Metabolic Alkalosis: High HCO₃⁻ levels (e.g., vomiting, diuretics).
2. Phosphate (H₂PO₄⁻) Buffer System
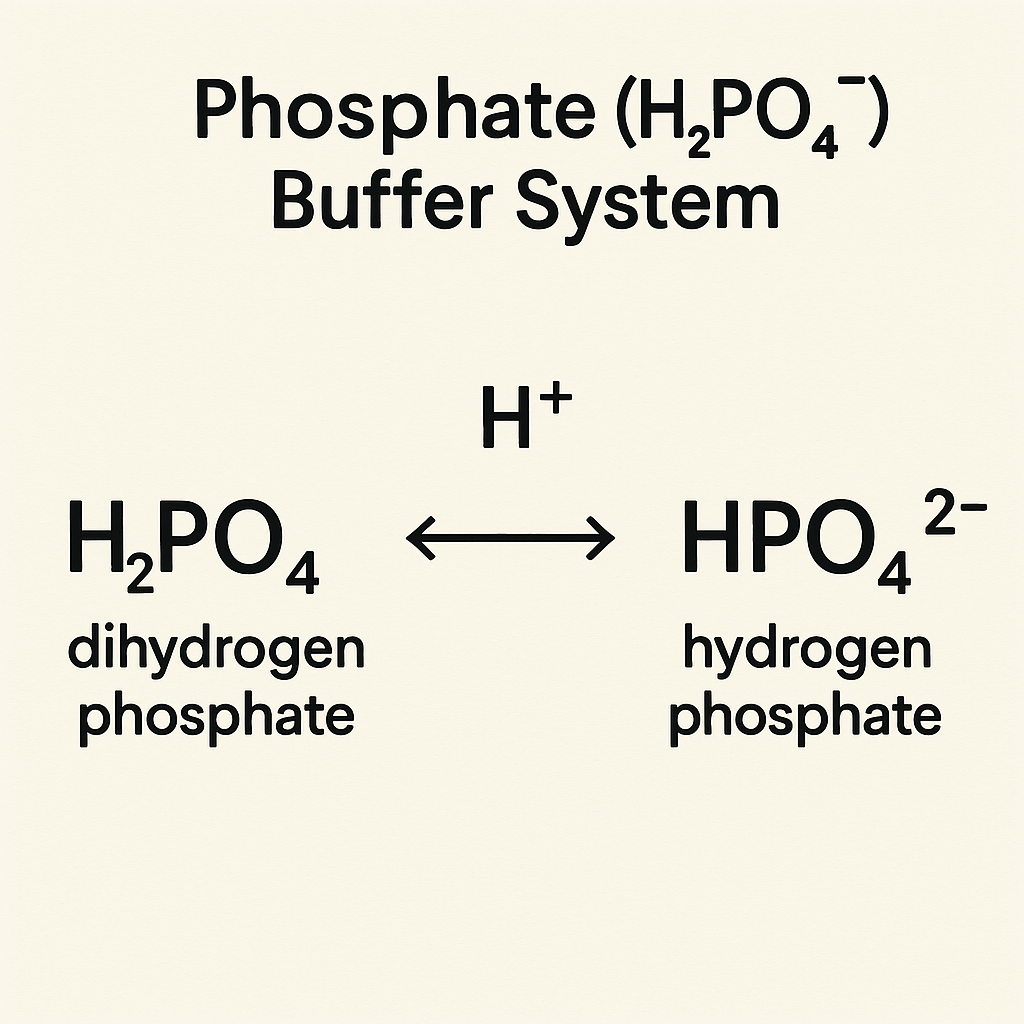
This buffer works intracellularly and in the renal system to regulate pH.
Reaction:
H2PO4−⇌H++HPO42−H_2PO_4^- \rightleftharpoons H^+ + HPO_4^{2-}H2PO4−⇌H++HPO42−
- When pH decreases (Acidosis):
- HPO₄²⁻ binds H⁺, forming H₂PO₄⁻, which is excreted in urine.
- When pH increases (Alkalosis):
- H₂PO₄⁻ releases H⁺, lowering pH.
Function in the Kidney
- Eliminates excess H⁺ in urine.
- Important for urine pH regulation.
Clinical Relevance
- Renal Acidosis: If kidney function is impaired, H⁺ excretion decreases, leading to acidosis.
3. Protein Buffer System (Hemoglobin & Plasma Proteins)
Proteins, especially hemoglobin and albumin, act as buffers in blood plasma and red blood cells (RBCs).
A. Hemoglobin (Hb) Buffer System
- Hemoglobin binds H⁺ and CO₂ in tissues and releases them in lungs.
- Works through the Bohr Effect:
- In acidic conditions (low pH), hemoglobin releases oxygen and binds H⁺.
- In alkaline conditions (high pH), hemoglobin binds oxygen and releases H⁺.
Reaction: Hb−H++O2⇌Hb−O2+H+Hb-H^+ + O_2 \rightleftharpoons Hb-O_2 + H^+Hb−H++O2⇌Hb−O2+H+
B. Plasma Proteins (Albumin, Globulins)
- Contain amino (-NH₂) and carboxyl (-COOH) groups that can accept or donate H⁺.
- Stabilize pH changes in plasma.
Clinical Importance
- Hemoglobin buffering is crucial in CO₂ transport.
- Protein buffering helps prevent drastic pH shifts in plasma.
4. Respiratory and Renal Regulation of Buffer Systems
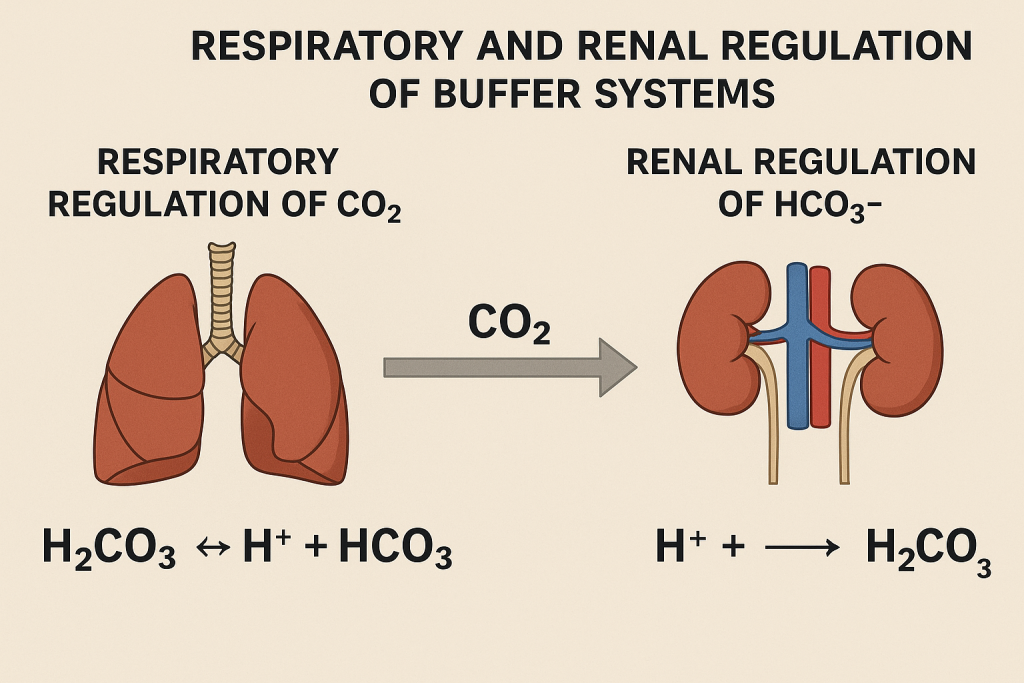
A. Respiratory System
- CO₂ is acidic and affects blood pH.
- Hypoventilation (slow breathing) retains CO₂ → ↓ pH (acidosis).
- Hyperventilation (rapid breathing) removes CO₂ → ↑ pH (alkalosis).
B. Renal System
- Kidneys regulate blood pH by controlling HCO₃⁻ and H⁺ excretion.
- In acidosis: Reabsorbs HCO₃⁻ and excretes H⁺.
- In alkalosis: Excretes HCO₃⁻ and retains H⁺.
5. Acid-Base Imbalances and Compensation
| Condition | Cause | pH | Compensation |
|---|---|---|---|
| Respiratory Acidosis | ↑ CO₂ retention (hypoventilation) | ↓ pH (<7.35) | Kidneys retain HCO₃⁻, excrete H⁺ |
| Respiratory Alkalosis | ↓ CO₂ (hyperventilation) | ↑ pH (>7.45) | Kidneys excrete HCO₃⁻, retain H⁺ |
| Metabolic Acidosis | Loss of HCO₃⁻ (diarrhea, renal failure) | ↓ pH (<7.35) | Lungs hyperventilate (↓ CO₂) |
| Metabolic Alkalosis | Loss of H⁺ (vomiting, diuretics) | ↑ pH (>7.45) | Lungs hypoventilate (↑ CO₂) |
6. Laboratory Tests for Blood Buffer Function
- Arterial Blood Gas (ABG) Test:
- pH: 7.35 – 7.45
- pCO₂: 35 – 45 mmHg
- HCO₃⁻: 22 – 26 mEq/L
- Serum Electrolytes:
- Measures Na⁺, K⁺, Cl⁻, HCO₃⁻ levels.
- Anion Gap Calculation: Anion Gap=(Na++K+)−(Cl−+HCO3−)\text{Anion Gap} = (\text{Na}^+ + \text{K}^+) – (\text{Cl}^- + \text{HCO}_3^-)Anion Gap=(Na++K+)−(Cl−+HCO3−)
- High anion gap acidosis → Lactic acidosis, ketoacidosis.
7. Nursing and Medical Interventions for Buffer Imbalance
A. Respiratory Acidosis (Low pH, High CO₂)
- Administer oxygen therapy.
- Improve ventilation (mechanical ventilation if needed).
- Treat underlying cause (COPD, pneumonia).
B. Respiratory Alkalosis (High pH, Low CO₂)
- Encourage slow breathing (paper bag method).
- Treat anxiety, pain, or fever.
- Monitor blood gases.
C. Metabolic Acidosis (Low pH, Low HCO₃⁻)
- Correct underlying cause (diabetes, renal failure).
- Administer IV sodium bicarbonate (if severe).
- Hydration and electrolyte correction.
D. Metabolic Alkalosis (High pH, High HCO₃⁻)
- Administer IV fluids (normal saline, potassium chloride).
- Correct underlying cause (vomiting, diuretic overuse).
- Monitor urine output and blood gases.
Respiratory and Renal Regulation of Blood pH
Introduction
The respiratory and renal systems play a crucial role in regulating blood pH (7.35 – 7.45) by maintaining the balance between acids (H⁺) and bases (HCO₃⁻). These systems work together to correct acid-base imbalances and ensure homeostasis.
- The respiratory system regulates pH by controlling CO₂ (carbon dioxide) levels through breathing.
- The renal system regulates pH by excreting hydrogen ions (H⁺) and reabsorbing bicarbonate (HCO₃⁻) in the kidneys.
Both systems function independently but compensate for each other when needed.
1. Respiratory Regulation of pH
The lungs regulate pH by controlling CO₂ (carbon dioxide) levels, which directly influence the bicarbonate (HCO₃⁻) buffer system.
Mechanism
- CO₂ dissolves in blood and forms carbonic acid (H₂CO₃): CO2+H2O⇌H2CO3⇌HCO3−+H+CO_2 + H_2O \rightleftharpoons H_2CO_3 \rightleftharpoons HCO_3^- + H^+CO2+H2O⇌H2CO3⇌HCO3−+H+
- Increased CO₂ → More H⁺ → Lower pH (Acidosis)
- Decreased CO₂ → Less H⁺ → Higher pH (Alkalosis)
Respiratory Compensation
- If pH drops (Acidosis):
- The lungs increase breathing rate (hyperventilation) to remove CO₂, raising pH.
- If pH rises (Alkalosis):
- The lungs slow breathing rate (hypoventilation) to retain CO₂, lowering pH.
2. Respiratory Acidosis (Low pH, High CO₂)
Causes:
- Hypoventilation (↓ breathing rate, CO₂ retention)
- Chronic obstructive pulmonary disease (COPD)
- Pneumonia, asthma
- Neuromuscular disorders (e.g., myasthenia gravis)
- Drug overdose (narcotics, sedatives)
Effects:
- Increased H⁺ (acid) → pH decreases (<7.35)
- Symptoms: Confusion, headache, drowsiness, rapid breathing (compensation attempt).
Compensation Mechanism:
- Renal compensation: Kidneys increase H⁺ excretion and HCO₃⁻ reabsorption.
Treatment:
- Improve ventilation (oxygen therapy, mechanical ventilation).
- Treat underlying cause (bronchodilators for COPD, antidotes for drug overdose).
3. Respiratory Alkalosis (High pH, Low CO₂)
Causes:
- Hyperventilation (fast breathing, excess CO₂ loss)
- Anxiety, panic attacks
- High altitude exposure
- Fever, sepsis
- Salicylate (aspirin) toxicity
Effects:
- Decreased H⁺ (low acid) → pH increases (>7.45)
- Symptoms: Dizziness, confusion, muscle cramps, tingling sensation.
Compensation Mechanism:
- Renal compensation: Kidneys increase HCO₃⁻ excretion and retain H⁺.
Treatment:
- Breathe into a paper bag (reintroduces CO₂).
- Treat underlying cause (sedation for anxiety, oxygen for altitude sickness).
4. Renal Regulation of pH
The kidneys regulate blood pH by controlling H⁺ and HCO₃⁻ levels in the urine.
Mechanism
- H⁺ Excretion:
- If blood pH is too low (acidosis): Kidneys excrete more H⁺.
- If blood pH is too high (alkalosis): Kidneys retain H⁺.
- HCO₃⁻ Reabsorption:
- In acidosis: Kidneys reabsorb more HCO₃⁻ to neutralize excess H⁺.
- In alkalosis: Kidneys excrete HCO₃⁻ to lower pH.
Location of Renal pH Regulation
- Proximal Tubule: Major site of HCO₃⁻ reabsorption.
- Distal Tubule & Collecting Duct: H⁺ is secreted into urine for acid removal.
5. Metabolic Acidosis (Low pH, Low HCO₃⁻)
Causes:
- Diabetic ketoacidosis (DKA)
- Lactic acidosis (shock, hypoxia)
- Renal failure (↓ acid excretion)
- Severe diarrhea (loss of HCO₃⁻)
Effects:
- Decreased HCO₃⁻ → Increased H⁺ → pH decreases (<7.35)
- Symptoms: Rapid breathing (Kussmaul respiration), confusion, fatigue, nausea.
Compensation Mechanism:
- Respiratory compensation: Lungs increase breathing rate to remove CO₂.
Treatment:
- IV bicarbonate if severe.
- Correct underlying cause (e.g., insulin for DKA, dialysis for kidney failure).
6. Metabolic Alkalosis (High pH, High HCO₃⁻)
Causes:
- Excess vomiting (loss of gastric acid)
- Diuretic overuse
- Excess bicarbonate intake (antacids)
- Cushing’s syndrome (↑ aldosterone, loss of H⁺)
Effects:
- Increased HCO₃⁻ → Decreased H⁺ → pH increases (>7.45)
- Symptoms: Muscle cramps, slow breathing, confusion, tetany.
Compensation Mechanism:
- Respiratory compensation: Lungs slow breathing rate (hypoventilation) to retain CO₂.
Treatment:
- Correct electrolyte imbalances (IV fluids, K⁺ supplementation).
- Stop diuretics or excess bicarbonate intake.
7. Interaction Between Respiratory and Renal Systems
| Condition | Cause | Primary Imbalance | Compensation |
|---|---|---|---|
| Respiratory Acidosis | CO₂ retention (hypoventilation) | ↓ pH, ↑ CO₂ | Kidneys retain HCO₃⁻, excrete H⁺ |
| Respiratory Alkalosis | CO₂ loss (hyperventilation) | ↑ pH, ↓ CO₂ | Kidneys excrete HCO₃⁻, retain H⁺ |
| Metabolic Acidosis | HCO₃⁻ loss (diarrhea, ketoacidosis) | ↓ pH, ↓ HCO₃⁻ | Lungs increase ventilation (↓ CO₂) |
| Metabolic Alkalosis | HCO₃⁻ retention (vomiting, diuretics) | ↑ pH, ↑ HCO₃⁻ | Lungs decrease ventilation (↑ CO₂) |
8. Laboratory Tests for Respiratory and Renal Function
- Arterial Blood Gas (ABG) Test:
- pH: 7.35 – 7.45
- pCO₂: 35 – 45 mmHg
- HCO₃⁻: 22 – 26 mEq/L
- Serum Electrolytes:
- Na⁺, K⁺, Cl⁻, HCO₃⁻ help assess pH imbalances.
- Urine pH Test:
- Normal range: 4.5 – 8.0.
- Acidic urine in metabolic acidosis, alkaline urine in metabolic alkalosis.
9. Nursing and Medical Management
- Monitor ABG reports and correct imbalances.
- Respiratory support for acidosis or alkalosis.
- IV fluids and electrolyte replacement for metabolic conditions.
- Dialysis for renal failure patients.
Arterial Blood Gas (ABG) Analysis: Normal Values, Abnormalities, and Interpretation
Introduction
Arterial Blood Gas (ABG) analysis is a critical test used to evaluate oxygenation, ventilation, and acid-base balance in the body. It helps diagnose and manage respiratory, metabolic, and acid-base disorders.
ABG provides measurements of:
- pH (Acidity or alkalinity of blood)
- Partial Pressure of Carbon Dioxide (pCO₂) (Respiratory function)
- Bicarbonate (HCO₃⁻) (Metabolic function)
- Partial Pressure of Oxygen (pO₂) (Oxygenation status)
- Oxygen Saturation (SaO₂) (Hemoglobin oxygen-binding capacity)
- Base Excess (BE) (Metabolic buffer capacity)
1. Normal ABG Values
| Parameter | Normal Range | Description |
|---|---|---|
| pH | 7.35 – 7.45 | Blood acidity or alkalinity |
| pCO₂ (Partial Pressure of Carbon Dioxide) | 35 – 45 mmHg | Reflects respiratory function |
| HCO₃⁻ (Bicarbonate) | 22 – 26 mEq/L | Reflects metabolic function |
| pO₂ (Partial Pressure of Oxygen) | 80 – 100 mmHg | Oxygen level in blood |
| SaO₂ (Oxygen Saturation) | 95 – 100% | Percentage of hemoglobin carrying oxygen |
| Base Excess (BE) | -2 to +2 mEq/L | Indicates metabolic acid-base status |
2. ABG Interpretation: Identifying Acid-Base Imbalances
The three main components of ABG analysis are:
- pH: Determines if the blood is acidic (<7.35) or alkaline (>7.45).
- pCO₂: Indicates respiratory contribution to acid-base balance.
- HCO₃⁻: Indicates metabolic contribution to acid-base balance.
A. Steps for ABG Interpretation
- Check pH:
- <7.35 → Acidosis
- >7.45 → Alkalosis
- 7.35 – 7.45 → Normal or Compensated
- Check pCO₂ (Respiratory Component):
- >45 mmHg → Respiratory Acidosis
- <35 mmHg → Respiratory Alkalosis
- Check HCO₃⁻ (Metabolic Component):
- <22 mEq/L → Metabolic Acidosis
- >26 mEq/L → Metabolic Alkalosis
- Determine Compensation:
- If pCO₂ or HCO₃⁻ is abnormal and pH is normal → Compensation is occurring.
- If pH remains abnormal → Uncompensated condition.
3. Abnormal ABG Findings and Their Causes
| Disorder | pH | pCO₂ | HCO₃⁻ | Causes |
|---|---|---|---|---|
| Respiratory Acidosis | ↓ (<7.35) | ↑ (>45 mmHg) | Normal or ↑ | Hypoventilation, COPD, Asthma, Pneumonia, Neuromuscular disorders, Drug overdose |
| Respiratory Alkalosis | ↑ (>7.45) | ↓ (<35 mmHg) | Normal or ↓ | Hyperventilation, Anxiety, Fever, High altitude, Pulmonary embolism |
| Metabolic Acidosis | ↓ (<7.35) | Normal or ↓ | ↓ (<22 mEq/L) | Diabetic ketoacidosis (DKA), Lactic acidosis, Renal failure, Severe diarrhea |
| Metabolic Alkalosis | ↑ (>7.45) | Normal or ↑ | ↑ (>26 mEq/L) | Vomiting, Excessive diuretic use, Antacid overdose, Hypokalemia |
4. Types of Compensation in ABG Analysis
When one system (respiratory or metabolic) is disturbed, the other compensates.
| Type of Compensation | pH | pCO₂ | HCO₃⁻ | Example |
|---|---|---|---|---|
| Uncompensated | Abnormal | Abnormal | Normal | Acute Respiratory Failure |
| Partially Compensated | Abnormal | Abnormal | Abnormal | Diabetic Ketoacidosis with Respiratory Response |
| Fully Compensated | Normal | Abnormal | Abnormal | Chronic COPD with Metabolic Adaptation |
A. Respiratory Compensation
- Lungs alter CO₂ levels to compensate for metabolic imbalances.
- Example: In metabolic acidosis, lungs increase breathing (hyperventilation) to expel CO₂ and raise pH.
B. Metabolic Compensation
- Kidneys adjust HCO₃⁻ levels to compensate for respiratory imbalances.
- Example: In respiratory acidosis, kidneys reabsorb more HCO₃⁻ to neutralize acidity.
5. Clinical Conditions Associated with ABG Abnormalities
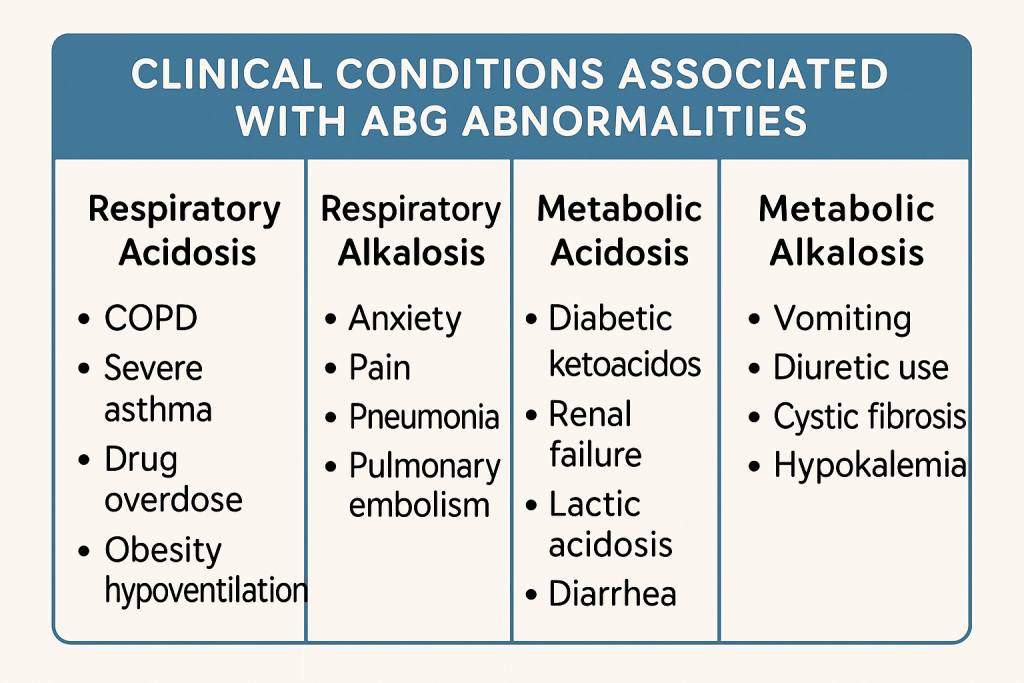
A. Respiratory Acidosis (pH < 7.35, pCO₂ > 45 mmHg)
Causes:
- Lung diseases: COPD, Asthma, Pneumonia.
- Airway obstruction: Foreign body, choking.
- Neuromuscular disorders: Myasthenia Gravis, Guillain-Barré Syndrome.
- Drug overdose: Opioids, sedatives.
Treatment:
- Improve ventilation (Oxygen therapy, CPAP, BiPAP).
- Treat underlying disease (bronchodilators, steroids).
B. Respiratory Alkalosis (pH > 7.45, pCO₂ < 35 mmHg)
Causes:
- Hyperventilation (Anxiety, Panic attacks).
- Fever, Sepsis, Pain.
- High altitude exposure.
- Salicylate (Aspirin) toxicity.
Treatment:
- Breathe into a paper bag (to retain CO₂).
- Treat anxiety (reassurance, benzodiazepines).
- Oxygen therapy for hypoxia.
C. Metabolic Acidosis (pH < 7.35, HCO₃⁻ < 22 mEq/L)
Causes:
- Diabetic Ketoacidosis (DKA).
- Lactic acidosis (Shock, Sepsis, Hypoxia).
- Renal failure (↓ Acid excretion).
- Severe diarrhea (Loss of HCO₃⁻).
Treatment:
- IV sodium bicarbonate (if severe).
- Fluids and electrolyte replacement.
- Dialysis for renal failure.
D. Metabolic Alkalosis (pH > 7.45, HCO₃⁻ > 26 mEq/L)
Causes:
- Vomiting, Gastric suctioning.
- Excessive diuretics (Furosemide, Thiazides).
- Antacid overdose (Bicarbonate overuse).
- Hypokalemia (Low K⁺ levels).
Treatment:
- IV fluids (Normal saline, Potassium replacement).
- Stop diuretics or antacid overuse.
- Monitor electrolyte levels.
6. How to Perform an ABG Test
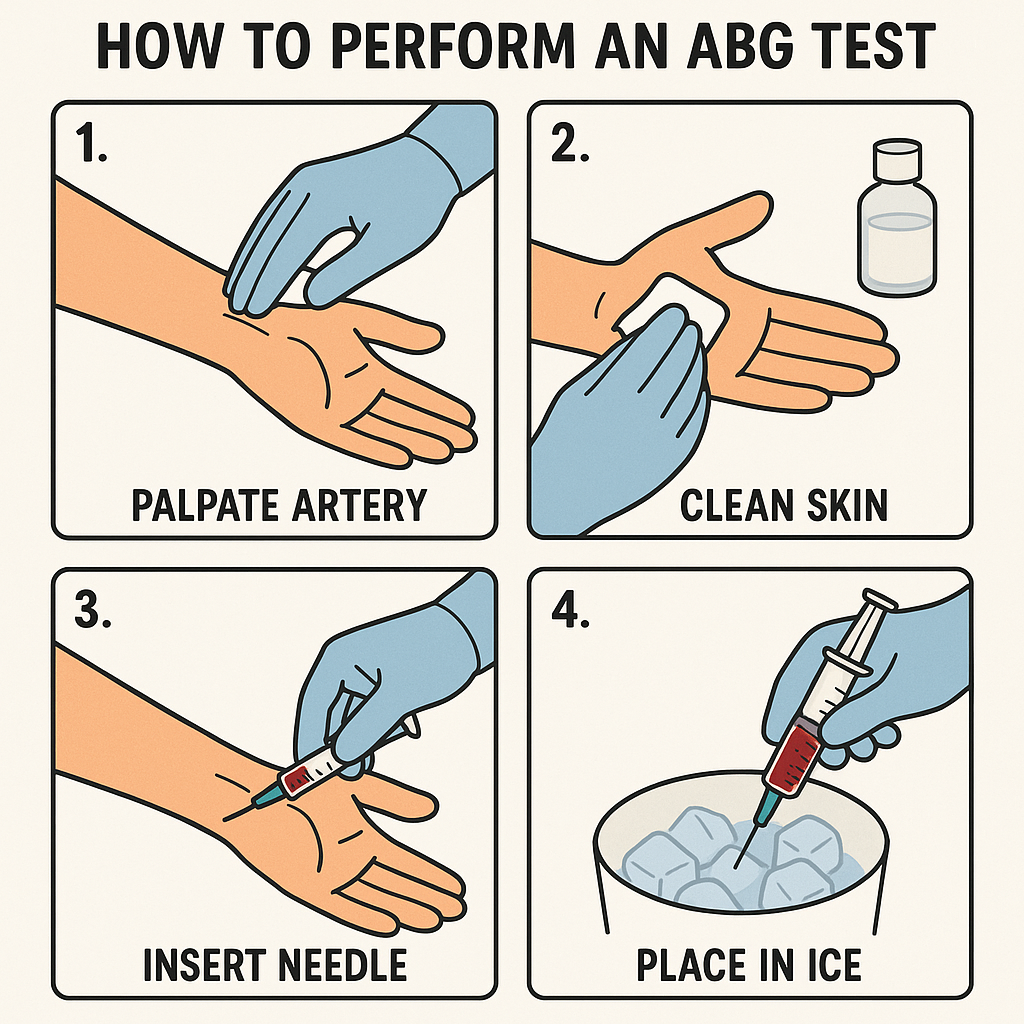
Procedure:
- Arterial blood sample is drawn from radial, femoral, or brachial artery.
- Sample is placed in heparinized syringe and kept on ice.
- Analyzed immediately for pH, pCO₂, pO₂, and HCO₃⁻ levels.
Precautions:
- Use Allen’s test to check radial artery patency.
- Avoid venous contamination.
- Interpret results in clinical context (consider oxygen therapy, ventilation status).
7. Conclusion
ABG analysis is essential for diagnosing acid-base disorders and monitoring critically ill patients. It helps identify respiratory vs. metabolic imbalances, determine compensation status, and guide treatment decisions.
Key Takeaways:
- pH determines acid-base status.
- pCO₂ indicates respiratory function.
- HCO₃⁻ reflects metabolic compensation.
- Compensation mechanisms must be assessed for full interpretation.
Timely ABG analysis and intervention can be life-saving in respiratory failure, metabolic disorders, and critical care patients.
Acid-Base Disorders:
Introduction
Acid-base disorders are conditions in which there is an imbalance in the blood pH due to excess acid (H⁺ ions) or base (HCO₃⁻ ions). These disorders can be classified as respiratory or metabolic, based on whether the cause is related to carbon dioxide (CO₂) retention/removal or bicarbonate (HCO₃⁻) imbalance.
Normal blood pH: 7.35 – 7.45
- Acidosis: pH < 7.35 (Excess acid or loss of base)
- Alkalosis: pH > 7.45 (Excess base or loss of acid)
Types of Acid-Base Disorders
| Disorder | Primary Imbalance | pH Level | Cause |
|---|---|---|---|
| Respiratory Acidosis | ↑ CO₂ retention | ↓ pH (<7.35) | Hypoventilation |
| Respiratory Alkalosis | ↓ CO₂ (excess loss) | ↑ pH (>7.45) | Hyperventilation |
| Metabolic Acidosis | ↓ HCO₃⁻ (bicarbonate loss) | ↓ pH (<7.35) | Ketoacidosis, Renal failure |
| Metabolic Alkalosis | ↑ HCO₃⁻ (excess base) | ↑ pH (>7.45) | Vomiting, Diuretics |
1. Respiratory Acidosis
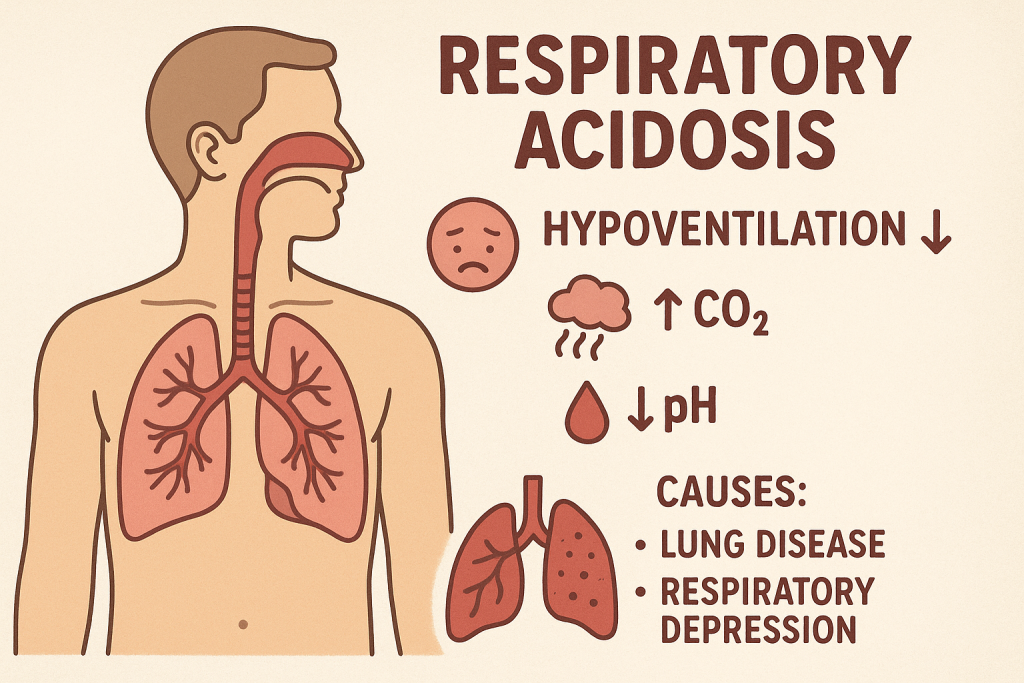
Definition:
A condition where CO₂ accumulates in the blood, leading to increased carbonic acid (H₂CO₃) and decreased pH.
Causes:
- Hypoventilation (↓ breathing rate)
- Chronic obstructive pulmonary disease (COPD)
- Asthma, Pneumonia
- Neuromuscular disorders (Myasthenia gravis, Guillain-Barré Syndrome)
- Drug overdose (narcotics, sedatives)
- Airway obstruction (choking, laryngospasm)
Signs & Symptoms:
- Neurological: Confusion, headache, drowsiness
- Respiratory: Slow, shallow breathing, cyanosis
- Cardiovascular: Hypotension, arrhythmias
Diagnosis:
- ABG Analysis:
- pH: <7.35
- pCO₂: >45 mmHg
- HCO₃⁻: Normal or increased (if compensated)
- Serum Electrolytes: Hyperkalemia (↑ K⁺)
- Chest X-ray, Pulmonary function test: Identifies lung disease
Medical Management:
- Improve ventilation (oxygen therapy, bronchodilators)
- Mechanical ventilation (if severe)
- Treat underlying cause (e.g., pneumonia, opioid overdose)
Nursing Management:
- Monitor respiratory rate, ABG results
- Encourage deep breathing exercises
- Maintain airway patency (suctioning if needed)
- Administer medications (bronchodilators, steroids)
2. Respiratory Alkalosis
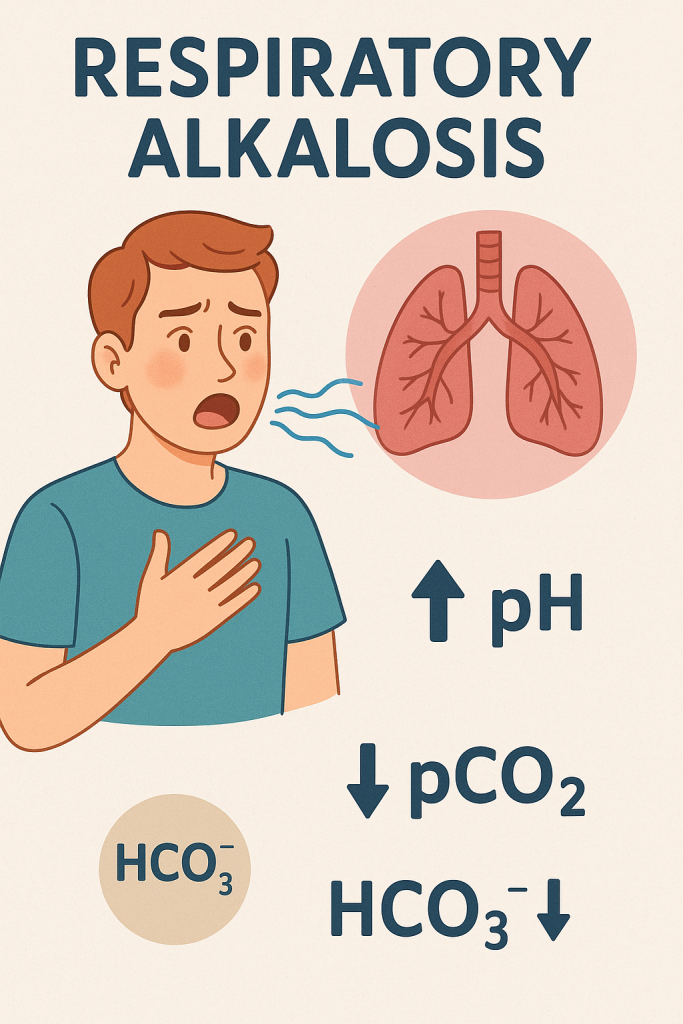
Definition:
A condition where excessive CO₂ is lost, leading to increased pH.
Causes:
- Hyperventilation (rapid breathing)
- Anxiety, panic attacks
- Fever, Sepsis
- Pain, Pregnancy
- High altitude exposure
- Salicylate (Aspirin) toxicity
Signs & Symptoms:
- Neurological: Dizziness, confusion, seizures
- Muscular: Tetany, muscle cramps, numbness
- Respiratory: Rapid, deep breathing
Diagnosis:
- ABG Analysis:
- pH: >7.45
- pCO₂: <35 mmHg
- HCO₃⁻: Normal or decreased (if compensated)
- Electrolytes: Hypokalemia (↓ K⁺), Hypocalcemia (↓ Ca²⁺)
Medical Management:
- Rebreathe CO₂ (paper bag method)
- Treat underlying cause (e.g., anxiety, fever)
- Sedatives for severe anxiety
Nursing Management:
- Monitor respiratory rate and mental status
- Encourage slow, controlled breathing
- Provide reassurance and calm environment
3. Metabolic Acidosis
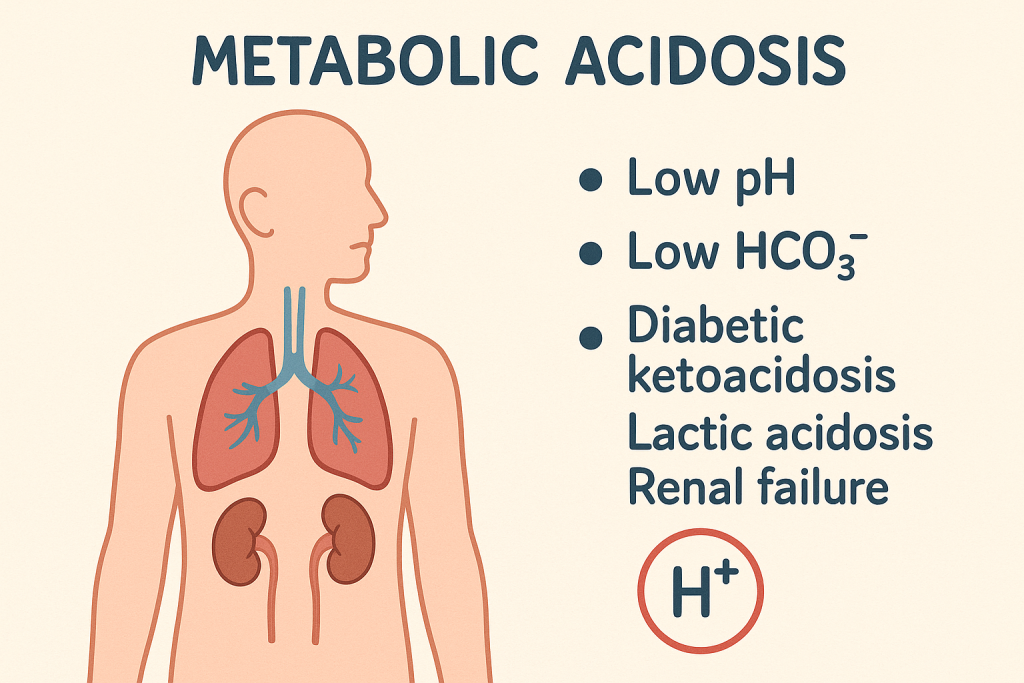
Definition:
A condition where excess acid accumulates or bicarbonate (HCO₃⁻) is lost, leading to decreased pH.
Causes:
- Diabetic Ketoacidosis (DKA)
- Lactic acidosis (shock, sepsis)
- Renal failure (↓ acid excretion)
- Severe diarrhea (loss of HCO₃⁻)
- Poisoning (Methanol, Aspirin overdose)
Signs & Symptoms:
- Neurological: Confusion, lethargy, headache
- Respiratory: Kussmaul’s respiration (deep, rapid breathing)
- Cardiovascular: Hypotension, arrhythmias
Diagnosis:
- ABG Analysis:
- pH: <7.35
- HCO₃⁻: <22 mEq/L
- pCO₂: Normal or decreased (if compensated)
- Anion Gap Calculation:
- High anion gap (>12 mEq/L) → Ketoacidosis, lactic acidosis
- Normal anion gap → Diarrhea, renal disease
Medical Management:
- IV sodium bicarbonate (if severe)
- Fluids and electrolyte correction
- Insulin therapy for DKA
- Dialysis for renal failure
Nursing Management:
- Monitor ABG, blood glucose, electrolyte levels
- Administer fluids and medications as prescribed
- Monitor respiratory effort (for Kussmaul’s breathing)
4. Metabolic Alkalosis
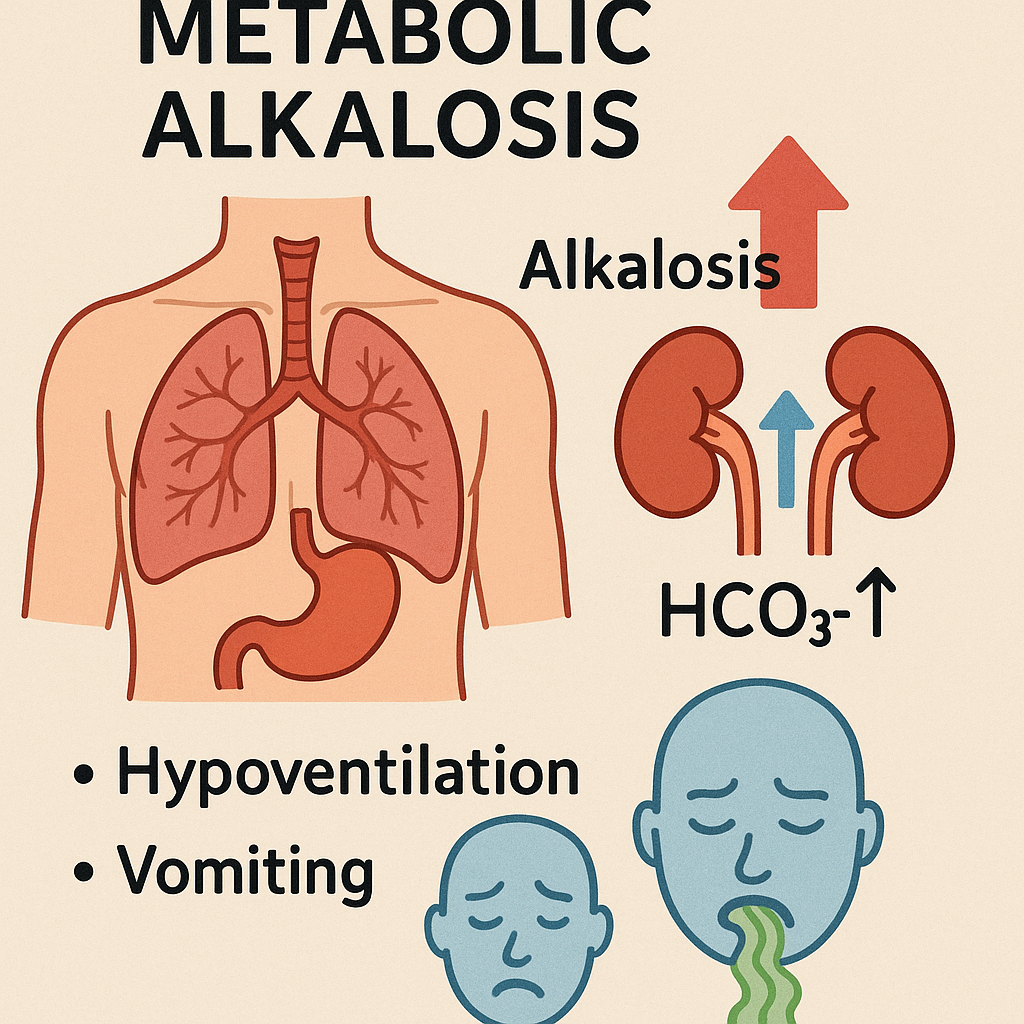
Definition:
A condition where bicarbonate (HCO₃⁻) increases or acid is lost, leading to increased pH.
Causes:
- Vomiting, Gastric suctioning
- Excessive diuretics (Furosemide, Thiazides)
- Antacid overdose (Excess bicarbonate)
- Hypokalemia (↓ K⁺ levels)
Signs & Symptoms:
- Neurological: Irritability, confusion
- Muscular: Muscle cramps, twitching, tetany
- Respiratory: Slow, shallow breathing
Diagnosis:
- ABG Analysis:
- pH: >7.45
- HCO₃⁻: >26 mEq/L
- pCO₂: Normal or increased (if compensated)
- Electrolytes: Hypokalemia (↓ K⁺), Hypocalcemia (↓ Ca²⁺)
Medical Management:
- IV fluids (normal saline, KCl)
- Stop diuretics or bicarbonate intake
- Acetazolamide (carbonic anhydrase inhibitor) to increase HCO₃⁻ excretion
Nursing Management:
- Monitor ABG, electrolyte levels
- Administer IV fluids and electrolytes
- Monitor neuromuscular function for tetany or cramps
Acid-Base Disorders: Compensatory Mechanisms
Introduction
The body maintains acid-base balance through compensatory mechanisms to keep the pH within the normal range (7.35 – 7.45). When an acid-base disorder occurs, the body attempts to compensate by adjusting either:
- Respiratory system (Lungs) → Regulates CO₂ (Carbon Dioxide)
- Renal system (Kidneys) → Regulates HCO₃⁻ (Bicarbonate) and H⁺ (Hydrogen Ions)
Compensation is classified into:
- Uncompensated → No compensation has occurred.
- Partially Compensated → Compensation has started, but pH is still abnormal.
- Fully Compensated → Compensation has restored pH to the normal range.
1. Respiratory Acidosis: Compensatory Mechanism
Cause: Hypoventilation → CO₂ Retention → ↓ pH (Acidic)
- Example: COPD, Asthma, Drug overdose, Neuromuscular disorders
Compensation: Renal Compensation (Slow, Hours to Days)
- Kidneys increase HCO₃⁻ reabsorption (to neutralize acid).
- Kidneys increase H⁺ excretion in urine.
- Urine becomes more acidic.
ABG Findings
| Stage | pH | pCO₂ | HCO₃⁻ |
|---|---|---|---|
| Uncompensated | ↓ (<7.35) | ↑ (>45 mmHg) | Normal (22-26 mEq/L) |
| Partially Compensated | ↓ (<7.35) | ↑ (>45 mmHg) | ↑ (>26 mEq/L) |
| Fully Compensated | Normal (7.35-7.45) | ↑ (>45 mmHg) | ↑ (>26 mEq/L) |
Example: A patient with COPD may have pH 7.36, pCO₂ 50 mmHg, HCO₃⁻ 30 mEq/L (fully compensated respiratory acidosis).
2. Respiratory Alkalosis: Compensatory Mechanism
Cause: Hyperventilation → CO₂ Loss → ↑ pH (Alkaline)
- Example: Anxiety, Fever, High altitude, Pain, Pulmonary embolism
Compensation: Renal Compensation (Slow, Hours to Days)
- Kidneys excrete more HCO₃⁻ in urine (to lower alkalinity).
- Kidneys retain more H⁺.
- Urine becomes more alkaline.
ABG Findings
| Stage | pH | pCO₂ | HCO₃⁻ |
|---|---|---|---|
| Uncompensated | ↑ (>7.45) | ↓ (<35 mmHg) | Normal (22-26 mEq/L) |
| Partially Compensated | ↑ (>7.45) | ↓ (<35 mmHg) | ↓ (<22 mEq/L) |
| Fully Compensated | Normal (7.35-7.45) | ↓ (<35 mmHg) | ↓ (<22 mEq/L) |
Example: A patient with hyperventilation due to anxiety may have pH 7.42, pCO₂ 28 mmHg, HCO₃⁻ 19 mEq/L (fully compensated respiratory alkalosis).
3. Metabolic Acidosis: Compensatory Mechanism
Cause: Excess Acid (H⁺) or Loss of HCO₃⁻ → ↓ pH (Acidic)
- Example: Diabetic ketoacidosis (DKA), Lactic acidosis, Renal failure, Diarrhea
Compensation: Respiratory Compensation (Fast, Minutes to Hours)
- Lungs increase ventilation (hyperventilation) to remove CO₂.
- “Kussmaul’s breathing” (deep, rapid breathing) occurs.
- This helps raise pH.
ABG Findings
| Stage | pH | pCO₂ | HCO₃⁻ |
|---|---|---|---|
| Uncompensated | ↓ (<7.35) | Normal (35-45 mmHg) | ↓ (<22 mEq/L) |
| Partially Compensated | ↓ (<7.35) | ↓ (<35 mmHg) | ↓ (<22 mEq/L) |
| Fully Compensated | Normal (7.35-7.45) | ↓ (<35 mmHg) | ↓ (<22 mEq/L) |
Example: A patient with diabetic ketoacidosis may have pH 7.38, pCO₂ 28 mmHg, HCO₃⁻ 18 mEq/L (fully compensated metabolic acidosis).
4. Metabolic Alkalosis: Compensatory Mechanism
Cause: Excess HCO₃⁻ or Loss of Acid (H⁺) → ↑ pH (Alkaline)
- Example: Vomiting, Gastric suctioning, Diuretic use, Excess antacids
Compensation: Respiratory Compensation (Fast, Minutes to Hours)
- Lungs slow breathing (hypoventilation) to retain CO₂.
- This lowers pH.
ABG Findings
| Stage | pH | pCO₂ | HCO₃⁻ |
|---|---|---|---|
| Uncompensated | ↑ (>7.45) | Normal (35-45 mmHg) | ↑ (>26 mEq/L) |
| Partially Compensated | ↑ (>7.45) | ↑ (>45 mmHg) | ↑ (>26 mEq/L) |
| Fully Compensated | Normal (7.35-7.45) | ↑ (>45 mmHg) | ↑ (>26 mEq/L) |
Example: A patient with severe vomiting may have pH 7.42, pCO₂ 47 mmHg, HCO₃⁻ 30 mEq/L (fully compensated metabolic alkalosis).
Summary of Compensation in Acid-Base Disorders
| Disorder | Primary Problem | Compensatory Mechanism | Organs Involved |
|---|---|---|---|
| Respiratory Acidosis | ↑ CO₂ (Hypoventilation) | ↑ HCO₃⁻ reabsorption, ↑ H⁺ excretion | Kidneys (Slow) |
| Respiratory Alkalosis | ↓ CO₂ (Hyperventilation) | ↓ HCO₃⁻ reabsorption, ↓ H⁺ excretion | Kidneys (Slow) |
| Metabolic Acidosis | ↓ HCO₃⁻ or ↑ H⁺ | Hyperventilation (↓ CO₂) | Lungs (Fast) |
| Metabolic Alkalosis | ↑ HCO₃⁻ or ↓ H⁺ | Hypoventilation (↑ CO₂) | Lungs (Fast) |
Key Takeaways
- Respiratory compensation is fast (minutes to hours) and involves lungs adjusting CO₂ levels.
- Metabolic compensation is slow (hours to days) and involves kidneys adjusting HCO₃⁻ levels.
- Compensation does not correct the underlying problem but helps stabilize pH.
- ABG analysis is essential to determine if compensation is uncompensated, partially compensated, or fully compensated.
Understanding compensatory mechanisms is critical for diagnosing and managing acid-base imbalances in clinical practice.