physio-B.sc-Unit-9-Renal system
🚰 Functions of the Kidney in Maintaining Homeostasis :-
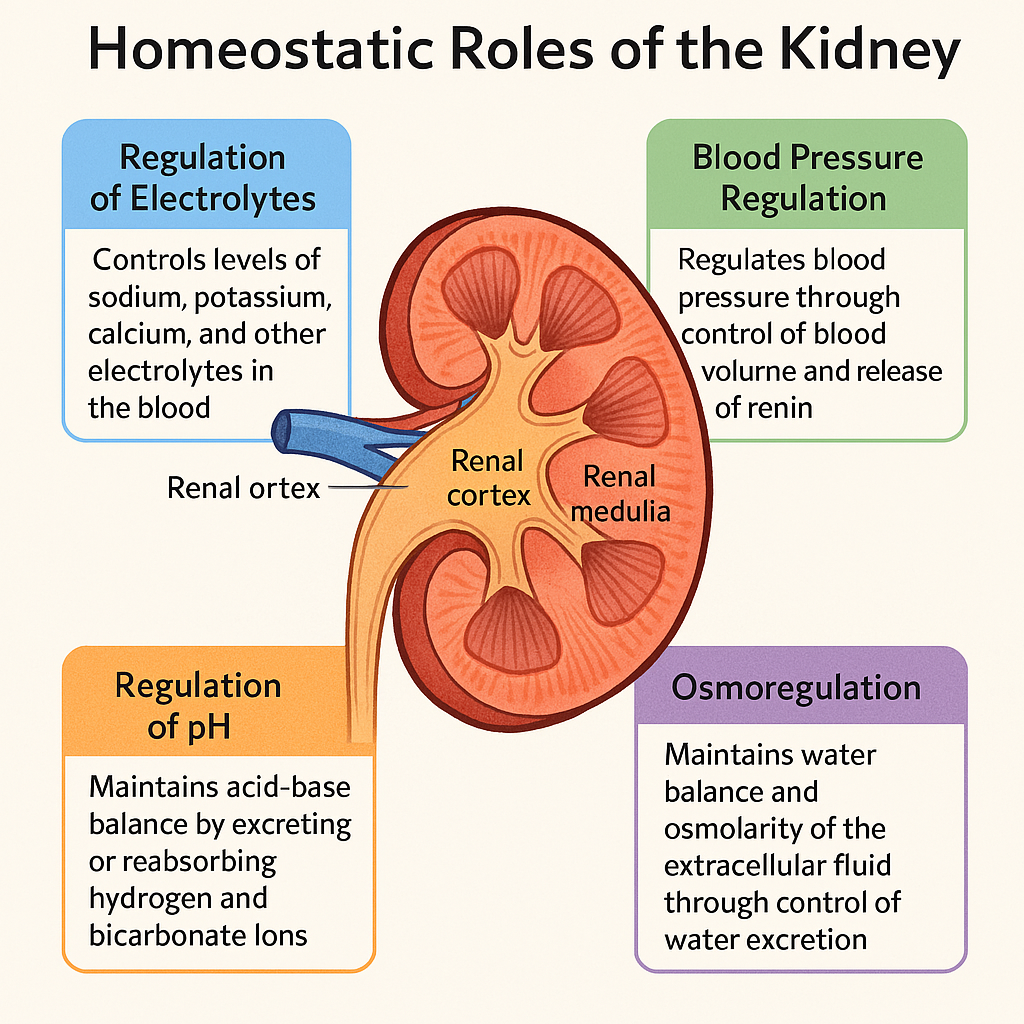
The kidneys are vital, bean-shaped organs responsible for maintaining internal fluid, electrolyte, and metabolic balance. Each kidney acts as a sophisticated filtration and regulatory unit, processing around 180 liters of blood-derived filtrate daily, and ensuring the stability of the body’s homeostatic environment through various tightly controlled physiological processes.
🧠 I. Major Homeostatic Functions of the Kidney
🔹 1. Regulation of Fluid and Electrolyte Balance
The kidneys help regulate:
- Water balance by adjusting the volume of water reabsorbed or excreted.
- Electrolytes, including sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), magnesium (Mg²⁺), phosphate (PO₄³⁻), and chloride (Cl⁻).
💧 Key mechanism:
- Antidiuretic hormone (ADH): Promotes water reabsorption in the distal nephron.
- Aldosterone: Enhances sodium reabsorption and potassium excretion.
🔍 Clinical note: Electrolyte disturbances can lead to arrhythmias, muscle cramps, or coma.
🔹 2. Regulation of Acid-Base Balance
The kidneys maintain the blood pH (7.35–7.45) by:
- Excreting hydrogen ions (H⁺)
- Reabsorbing bicarbonate (HCO₃⁻)
🧪 Key mechanisms:
- Renal tubules secrete H⁺ and generate new bicarbonate.
- Ammonia (NH₃) acts as a buffer in the distal nephron.
🔍 Important for managing metabolic acidosis or alkalosis.
🔹 3. Excretion of Metabolic Wastes and Toxins
Kidneys remove:
- Nitrogenous wastes (e.g., urea from protein metabolism, creatinine, uric acid)
- Drugs and toxins
- Excess water and solutes
🗑️ Process:
Filtration at the glomerulus → secretion and reabsorption → excretion via urine.
🔍 In renal failure, waste accumulation leads to uremia.
🔹 4. Regulation of Blood Pressure
The kidneys play a key role via the Renin–Angiotensin–Aldosterone System (RAAS):
- Renin, released by juxtaglomerular cells, initiates a cascade:
- Angiotensin I → Angiotensin II (a potent vasoconstrictor)
- Stimulates aldosterone → Na⁺ and water retention
- Outcome: ↑ blood volume and pressure
🔍 Dysregulation leads to hypertension or hypotension.
🔹 5. Erythropoiesis Regulation
The kidney produces erythropoietin (EPO) in response to hypoxia:
- EPO stimulates red blood cell (RBC) production in the bone marrow.
🔍 In chronic kidney disease (CKD), EPO deficiency causes anemia.
🔹 6. Regulation of Calcium and Phosphate Balance
The kidneys regulate:
- Phosphate excretion
- Activation of vitamin D (calcitriol), which increases calcium absorption from the gut.
🔍 Failure to do so leads to hypocalcemia and renal osteodystrophy.
🔹 7. Detoxification and Hormone Degradation
- The kidney breaks down insulin, parathyroid hormone, and other circulating substances.
- Assists the liver in detoxification by excreting conjugated metabolites.
🧬 II. Summary of Kidney Functions in Homeostasis
| Homeostatic Role | Function |
|---|---|
| Fluid balance | Adjusts water output based on hydration status |
| Electrolyte regulation | Maintains levels of Na⁺, K⁺, Ca²⁺, Mg²⁺, Cl⁻, PO₄³⁻ |
| Acid-base balance | Excretes H⁺, conserves HCO₃⁻ to stabilize blood pH |
| Waste removal | Filters and eliminates urea, creatinine, drugs, toxins |
| Blood pressure control | Activates RAAS, controls fluid retention and vascular resistance |
| RBC production | Secretes erythropoietin to stimulate bone marrow |
| Vitamin D activation | Converts inactive vitamin D to active form for Ca²⁺ homeostasis |
🩺 III. Clinical Relevance
- Renal failure affects nearly all homeostatic functions → requires dialysis or transplantation.
- Urinalysis and serum creatinine are essential for assessing renal function.
- Kidney dysfunction impacts BP control, anemia management, and electrolyte balance.
The kidneys are central to maintaining internal equilibrium, regulating fluid, electrolyte, and acid-base status, while also supporting blood pressure control, red blood cell production, and waste excretion. Their diverse and intricate roles make them indispensable for homeostasis, and any compromise in their function has multisystem consequences.
🩺 Glomerular Filtration Rate (GFR) – Academic Explanation
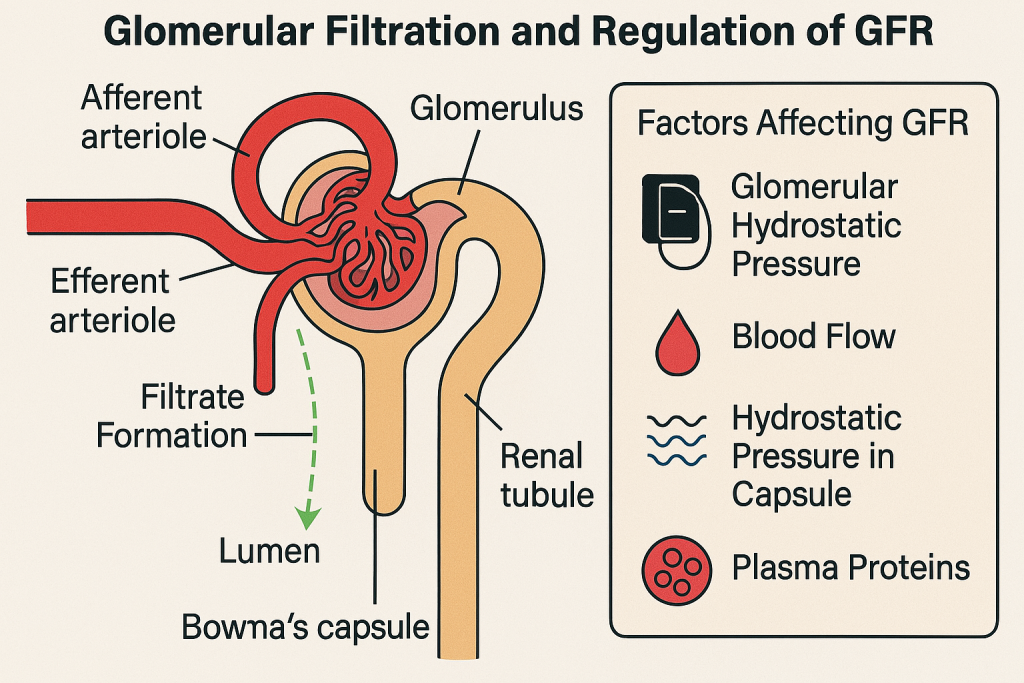
🔬 Definition
GFR is the rate at which plasma is filtered through the glomeruli of the kidneys into the Bowman’s capsule per unit time. It is a key indicator of kidney function and is essential for assessing the efficiency of waste removal from the blood.
- Normal GFR (adult): ~ 90–120 mL/min/1.73 m²
- Falls with age, renal diseases, dehydration, or hypotension
🧠 I. Anatomical and Physiological Basis of GFR
Filtration occurs in the renal corpuscle, which consists of:
- Glomerulus – a tuft of capillaries
- Bowman’s capsule – surrounds the glomerulus
The filtration barrier is composed of:
- Fenestrated endothelium of glomerular capillaries
- Basement membrane
- Podocytes with filtration slits
Only small molecules like water, glucose, ions, urea, and amino acids pass through. Proteins and cells are retained in the blood.
⚙️ II. Determinants of GFR
GFR is influenced by Starling forces (pressure gradients):
Net Filtration Pressure (NFP) =
(Glomerular hydrostatic pressure) – (Bowman’s capsule hydrostatic pressure + Glomerular oncotic pressure)
- Glomerular hydrostatic pressure (P_gc)
- ~55 mmHg; pushes fluid out of capillaries
- Bowman’s capsule pressure (P_bs)
- ~15 mmHg; opposes filtration
- Glomerular oncotic pressure (π_gc)
- ~30 mmHg; pulls water back into capillaries
Typical Net Filtration Pressure: ~10 mmHg
🧪 III. Measurement and Estimation of GFR
1. Direct Measurement
- Uses substances that are freely filtered and neither secreted nor reabsorbed.
- Inulin clearance is the gold standard but impractical clinically.
2. Clinical Estimation
- Based on serum creatinine levels (endogenous marker)
- Equations used:
- eGFR (CKD-EPI or MDRD formula) – adjusts for age, sex, body size, race
- Creatinine clearance via 24-hour urine
📉 IV. GFR Values and Kidney Function
| GFR (mL/min/1.73m²) | Stage | Clinical Implication |
|---|---|---|
| >90 | Normal or high | Healthy or early kidney disease |
| 60–89 | Mild decrease | Stage 2 CKD |
| 30–59 | Moderate decrease | Stage 3 CKD |
| 15–29 | Severe decrease | Stage 4 CKD |
| <15 | Kidney failure | Stage 5 CKD / End-stage renal disease |
🧬 V. Regulation of GFR
A. Autoregulation
- Myogenic mechanism – afferent arterioles constrict/dilate based on pressure changes.
- Tubuloglomerular feedback – macula densa senses NaCl and adjusts afferent tone.
B. Hormonal and Neural Regulation
- Sympathetic nervous system: vasoconstriction → ↓ GFR
- Angiotensin II: constricts efferent arteriole → maintains GFR
- Atrial natriuretic peptide (ANP): dilates afferent arteriole → ↑ GFR
⚠️ VI. Factors Reducing GFR
- Hypotension or shock
- Renal artery stenosis
- Glomerulonephritis
- Dehydration
- Diabetes and hypertension-induced nephropathy
- Nephrotoxic drugs (e.g., NSAIDs, aminoglycosides)
🩺 VII. Clinical Significance of GFR
- Early detection of kidney disease
- Monitoring progression of chronic kidney disease (CKD)
- Adjusting medication dosages (e.g., in renal impairment)
- Evaluating renal transplant function
- Assessing effects of therapies on kidney function
GFR reflects the filtration capacity of the kidneys and is fundamental to maintaining fluid, electrolyte, and toxin balance in the body. It serves as a cornerstone for diagnosing and managing renal disorders, making it a vital concept for all health professionals.
🚶♂️ Functions of the Ureters –
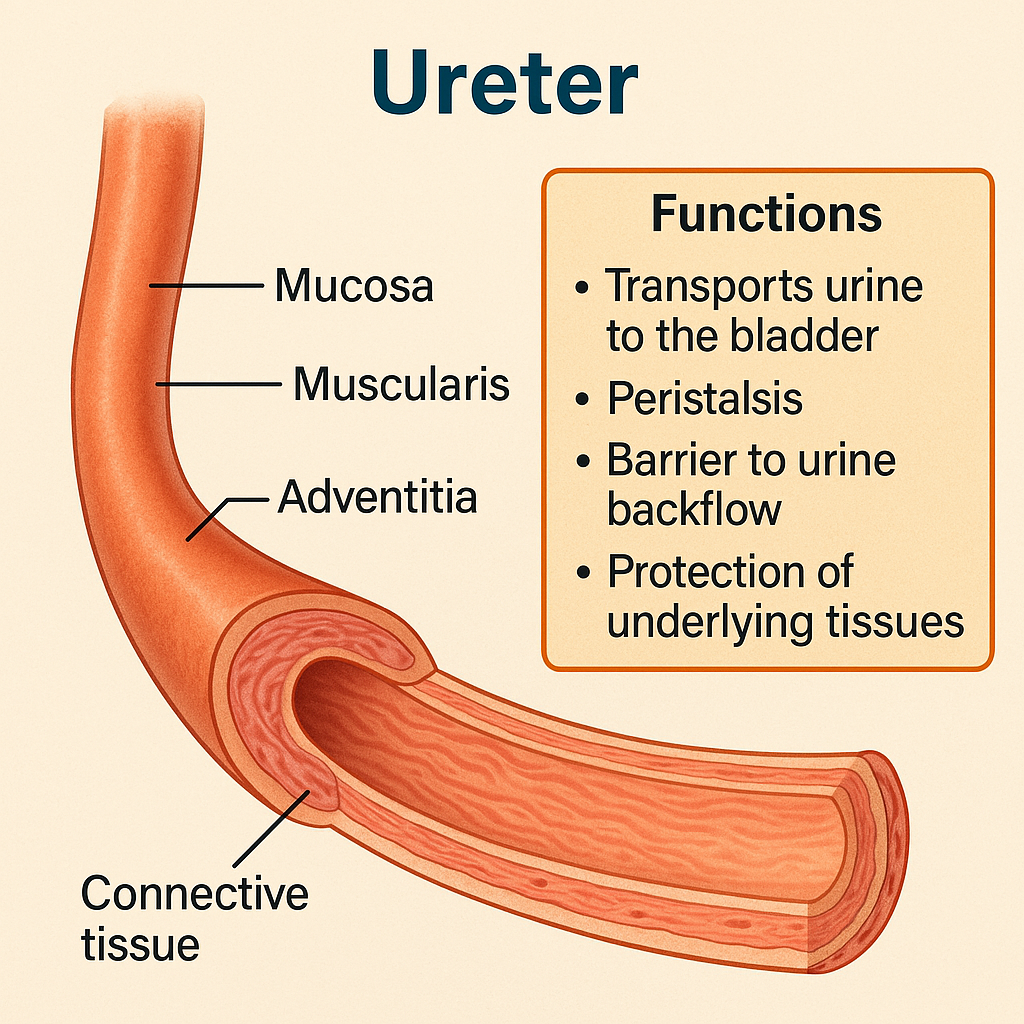
The ureters are a pair of narrow, muscular tubes that transport urine from the kidneys to the urinary bladder. Each ureter is approximately 25–30 cm long and extends from the renal pelvis to the posterior surface of the bladder. Despite their seemingly simple role, ureters possess specialized structural and functional properties that ensure unidirectional, efficient, and regulated urine transport.
🔬 I. Structural Overview of the Ureter
Each ureter has three layers:
- Mucosa
- Lined by transitional epithelium (urothelium) that can stretch
- Supported by a lamina propria (connective tissue)
- Muscularis
- Consists of smooth muscle layers:
- Inner longitudinal
- Outer circular (with a third outer longitudinal layer in the lower third)
- Responsible for peristaltic contractions
- Consists of smooth muscle layers:
- Adventitia
- Outer connective tissue layer
- Anchors the ureter in place and contains blood vessels, nerves, and lymphatics
✅ II. Main Functions of the Ureters
🔹 1. Urine Transport
- Primary function: Carry urine from the renal pelvis to the urinary bladder
- Achieved by peristalsis, not gravity alone
- Peristaltic waves occur every 10–15 seconds, triggered by renal pelvis stretch receptors
- Ensures continuous flow of urine even when lying down or in low-pressure situations
🔹 2. Peristaltic Propulsion
- Ureters possess intrinsic pacemaker activity in smooth muscle
- Coordinated waves of contraction move urine distally
- Speed and frequency of peristalsis adapt based on:
- Volume of urine
- Hydration status
- Neural and hormonal inputs (e.g., autonomic nervous system)
🩺 Clinical note: Obstruction or dysfunction can impair peristalsis, causing hydronephrosis or renal colic.
🔹 3. Unidirectional Flow – Anti-Reflux Mechanism
- At the junction with the bladder, the ureter passes obliquely through the bladder wall
- This creates a valve-like mechanism that prevents backflow (vesicoureteral reflux) during bladder contraction
- Essential to protect kidneys from:
- Infection (ascending UTIs)
- High bladder pressure
🔹 4. Protection from Urine Toxicity
- Transitional epithelium forms a tight barrier against the reabsorption of toxic waste products
- Urothelium also resists stretch and chemical irritation caused by varying urine composition
🔹 5. Sensory Function
- Ureters are richly innervated by sensory afferent fibers
- These detect distension, inflammation, or stone impaction
- Activation of nociceptors (pain receptors) leads to ureteric colic, a hallmark of kidney stones
🧠 III. Regulation of Ureteral Function
- Controlled by autonomic nervous system:
- Sympathetic stimulation tends to inhibit ureteral contractions
- Parasympathetic stimulation enhances peristalsis
- Hormones like ADH and aldosterone affect urine volume and indirectly impact ureteral load
🧾 IV. Clinical Significance
| Condition | Impact on Ureter Function |
|---|---|
| Ureteric stones | Block peristalsis, cause pain and hydronephrosis |
| Neurogenic bladder | Can impair ureter-bladder coordination |
| Vesicoureteral reflux | Causes backflow of urine → risk of pyelonephritis |
| Strictures/tumors | Physically obstruct urine flow |
| Ureteral injury | Can occur during pelvic surgery → urine leakage |
The ureters are vital conduits that actively transport urine using peristaltic motion, maintain unidirectional flow, and prevent renal damage from pressure or infection. Their efficient function is essential for urinary system integrity and overall homeostasis. Disruption of ureteral function can result in significant clinical consequences, making their understanding crucial in nursing and medical care.
🫙 Urinary Bladder – Functions
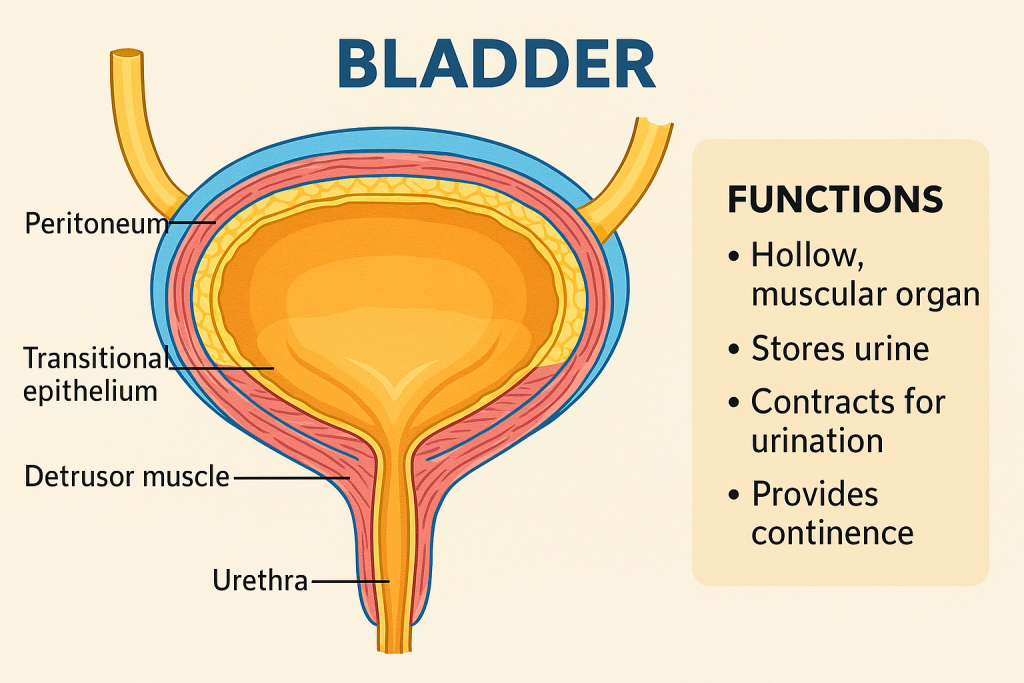
The urinary bladder is a hollow, muscular, distensible organ that functions as a temporary storage reservoir for urine. It plays a central role in the urinary system, working in coordination with the kidneys, ureters, urethra, and associated neural mechanisms to regulate urine collection, storage, and controlled elimination (micturition).
🔬 I. Anatomy and Structural Basis
- Location: In the pelvic cavity, posterior to the pubic symphysis.
- Layers:
- Mucosa (transitional epithelium – allows stretching)
- Submucosa (connective tissue and blood supply)
- Muscularis (Detrusor muscle) – smooth muscle for contraction
- Serosa or adventitia (outer covering)
- Trigone: A smooth triangular area at the bladder base between the openings of the two ureters and the urethra – sensitive to stretch.
- Innervation:
- Parasympathetic (S2–S4): Stimulates bladder contraction.
- Sympathetic (T11–L2): Promotes bladder relaxation and internal sphincter contraction.
- Somatic (pudendal nerve): Controls external urethral sphincter voluntarily.
🧠 II. Key Functions of the Urinary Bladder
🔹 1. Urine Storage
- The bladder stores urine from the kidneys, delivered via the ureters.
- Its high compliance and stretchable wall allow it to hold up to 400–600 mL in adults.
- Transitional epithelium and rugae allow expansion without increasing internal pressure.
🩺 Clinical relevance: Overdistension can lead to detrusor muscle dysfunction and urinary retention.
🔹 2. Urine Expulsion (Micturition)
- The bladder actively participates in micturition, the process of urination.
- Triggered when bladder volume reaches ~250–400 mL.
- Stretch receptors in the bladder wall send signals to the brainstem (pontine micturition center).
- Coordinated contraction of the detrusor muscle and relaxation of the internal and external sphincters lead to urine expulsion.
🧠 Voluntary control is developed by age 2–3, involving cortical inhibition of the reflex arc.
🔹 3. Maintenance of Continence
- Continence is maintained by:
- Internal urethral sphincter (smooth muscle – involuntary)
- External urethral sphincter (skeletal muscle – voluntary)
- Proper bladder function ensures social continence, avoiding involuntary leakage.
🩺 Clinical relevance: Disorders like stress incontinence, urge incontinence, or neurogenic bladder can disrupt this balance.
🔹 4. Protection Against Infection and Reflux
- Regular bladder emptying helps flush pathogens, reducing UTI risk.
- Proper valve-like entry of ureters into the bladder wall prevents backflow (vesicoureteral reflux).
- Mucosal lining contains uroplakins and glycosaminoglycans, forming a barrier against bacteria.
🔹 5. Pressure Regulation and Coordination
- The bladder functions as a low-pressure reservoir, even during filling.
- The detrusor muscle remains relaxed due to sympathetic inhibition until the need to void arises.
- At the point of voiding, parasympathetic signals activate contraction, ensuring complete emptying.
🩺 III. Clinical and Nursing Significance
- Bladder function assessment is essential in:
- Post-operative care (e.g., urinary retention)
- Neurological conditions (e.g., spinal cord injury, multiple sclerosis)
- Elderly care (e.g., overactive bladder, incontinence)
- Nursing interventions include:
- Bladder training
- Monitoring input/output
- Intermittent catheterization
- Patient education on pelvic floor exercises
🧾 IV. Summary of Bladder Functions
- Stores urine safely until voluntary voiding
- Expels urine efficiently through micturition
- Maintains continence with sphincter control
- Protects kidneys from reflux and infection
- Coordinates neural and muscular systems for regulated output
The urinary bladder is a complex organ with dynamic roles in urine storage, elimination, continence, and protection. Understanding its physiology is vital for managing urinary disorders, performing catheterization, and maintaining fluid balance and hygiene in clinical practice.
🚻 Urethra – Structure and Functions: Academic Overview
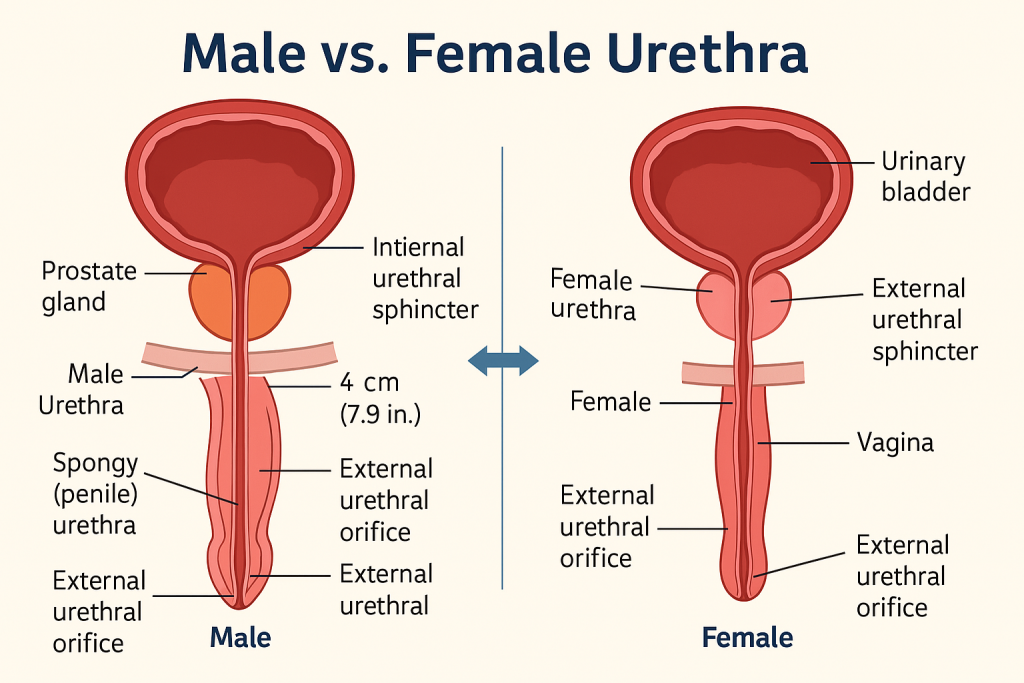
The urethra is a muscular tubular structure that conveys urine from the urinary bladder to the outside of the body during micturition. In males, it also serves as a passage for semen, making it a part of both the urinary and reproductive systems.
🔬 I. Structural Anatomy of the Urethra
🔹 A. In Females
- Length: ~4 cm
- Location: Extends from the bladder neck to the external urethral orifice in the vulva, anterior to the vaginal opening.
- Structure: Short, straight, and embedded in the anterior vaginal wall.
- Lining: Transitional epithelium (proximal) → stratified squamous epithelium (distal)
🔹 B. In Males
- Length: ~18–20 cm
- Structure: Divided into 3 segments:
- Prostatic urethra – passes through prostate gland
- Membranous urethra – shortest and narrowest; passes through urogenital diaphragm
- Spongy (penile) urethra – passes through corpus spongiosum of penis
- Lining: Similar transitional to pseudostratified columnar, ending with stratified squamous epithelium
The external urethral sphincter (voluntary) and internal sphincter (involuntary, mainly in males) regulate the flow of urine.
🧬 II. Properties of the Urethra
- Elasticity and Flexibility – Allows distension during urination or ejaculation (in males).
- Lined with Epithelium – Protects from acidic urine and potential pathogens.
- Innervated – Rich autonomic and somatic innervation allows reflex and voluntary control.
- Associated with Sphincters – Facilitates continence and controlled voiding.
🧠 III. Functions of the Urethra
✅ 1. Urinary Excretion (in Both Sexes)
- Acts as a conduit for urine to exit the body from the bladder.
- Coordinated with detrusor muscle contraction and sphincter relaxation.
✅ 2. Reproductive Function (Males Only)
- In males, also serves as a passage for semen during ejaculation.
- Synchronized with ejaculation reflex to prevent urine and semen mixing.
✅ 3. Maintenance of Continence
- External urethral sphincter (striated muscle): Provides voluntary control over urination.
- Internal sphincter (smooth muscle, in males): Prevents retrograde ejaculation and supports continence.
✅ 4. Defense Against Infection
- Urethral mucosa secretes mucus and antimicrobial peptides (e.g., defensins).
- Regular voiding flushes out pathogens from the urinary tract.
🩺 IV. Clinical Considerations
| Condition | Impact on Function |
|---|---|
| Urethritis | Inflammation, often due to infection (e.g., STIs) |
| Urinary tract infection | More common in females due to short urethra |
| Strictures | Narrowing, often from trauma or infection → urinary retention |
| Incontinence | Weakness of sphincters → loss of bladder control |
| Prostate enlargement | Obstructs male urethra, causing difficulty in urination |
| Catheterization | Requires knowledge of urethral anatomy to avoid trauma |
The urethra is a vital anatomical structure essential for urinary excretion, reproductive function (in males), and maintaining continence. Despite its small size, it plays a significant role in urinary physiology and infection defense. Knowledge of its structure and functions is crucial in nursing for catheterization, UTI prevention, and continence care.
🚽 Micturition – Physiology
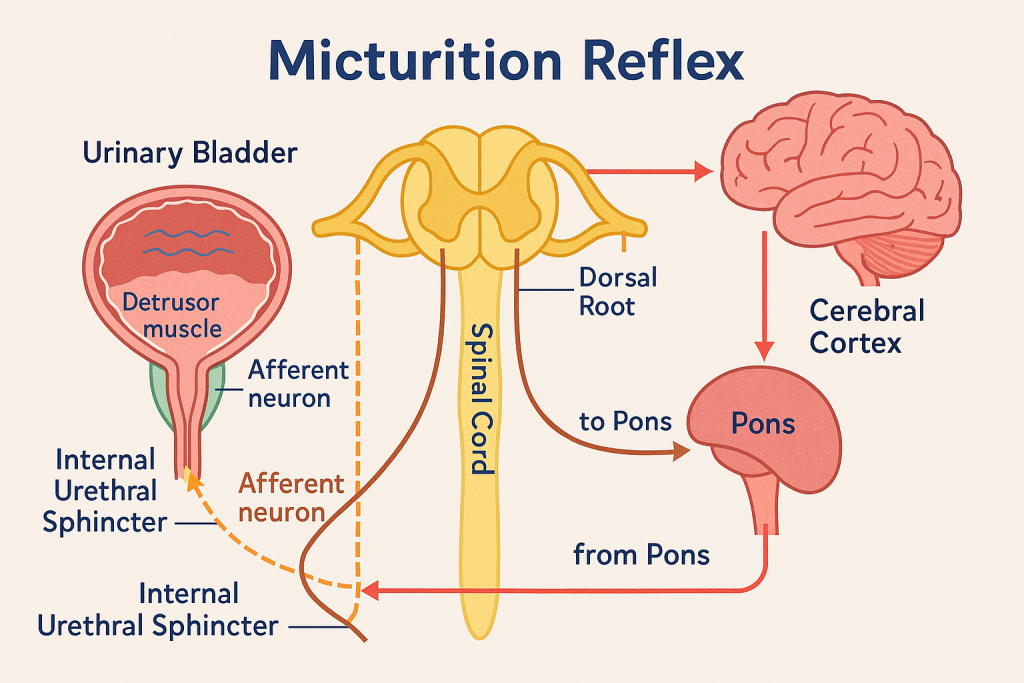
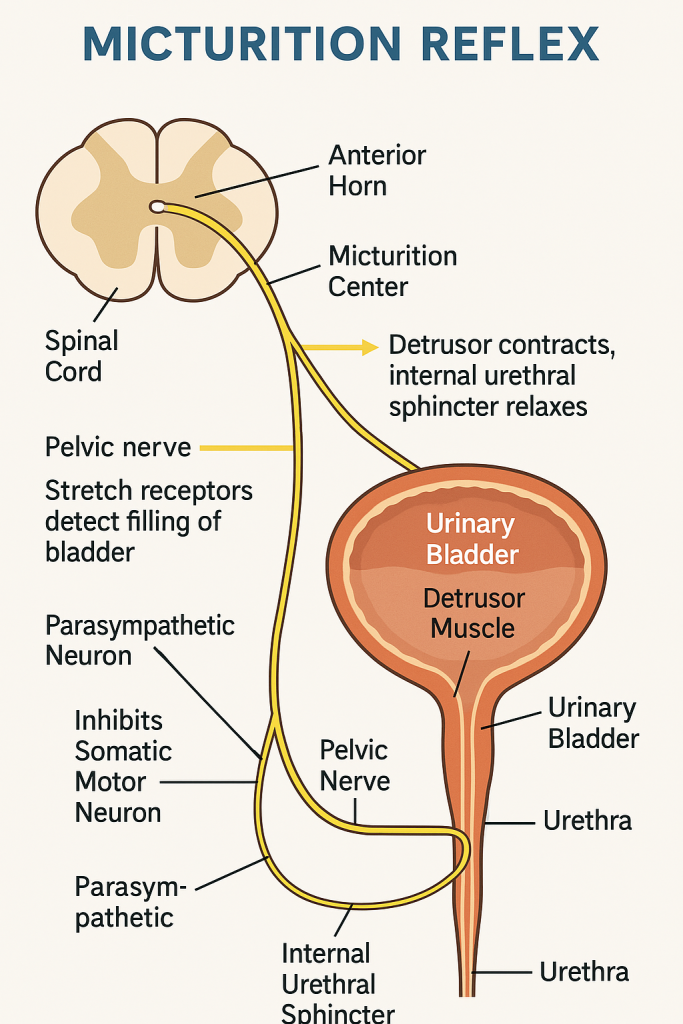
Micturition refers to the process by which urine is expelled from the urinary bladder through the urethra. It involves a complex interaction between nervous control, smooth and skeletal muscle coordination, and urinary tract anatomy.
🧠 I. Anatomy Involved in Micturition
- Kidneys – Produce urine
- Ureters – Transport urine to the bladder
- Urinary Bladder – Temporary reservoir of urine
- Urethra – Conducts urine out of the body
Bladder Wall:
- Lined with detrusor muscle (smooth muscle)
- Contains stretch receptors sensitive to volume changes
Sphincters:
- Internal urethral sphincter (involuntary, smooth muscle)
- External urethral sphincter (voluntary, skeletal muscle under somatic control)
🔬 II. Phases of Micturition
🔹 A. Urine Storage Phase (Filling Phase)
- Bladder fills with urine slowly (~400–600 mL capacity)
- Stretch receptors in the bladder wall begin to activate as volume increases
- Sympathetic nervous system (hypogastric nerve, T11–L2) maintains:
- Relaxation of detrusor muscle (via β-adrenergic receptors)
- Contraction of internal urethral sphincter (via α-adrenergic receptors)
- Somatic nervous system (pudendal nerve, S2–S4) maintains tonic contraction of the external sphincter
🧠 Result: Bladder stores urine without involuntary leakage
🔹 B. Voiding Phase (Emptying Phase)
Once a critical volume (~250–300 mL) is reached:
- Stretch receptors send signals to the pontine micturition center (PMC) via the pelvic nerves
- If voiding is appropriate (under voluntary control), the PMC:
- Inhibits sympathetic and somatic outflow
- Stimulates parasympathetic nerves (S2–S4) → causes:
- Detrusor muscle contraction
- Relaxation of internal urethral sphincter
- Voluntary relaxation of external sphincter allows urine to flow
🧠 Result: Coordinated contraction of bladder + sphincter relaxation = urination
🧬 III. Nervous System Control
| System | Nerve Pathway | Action |
|---|---|---|
| Parasympathetic | Pelvic nerve (S2–S4) | Contracts detrusor, relaxes internal sphincter |
| Sympathetic | Hypogastric nerve (T11–L2) | Relaxes detrusor, contracts internal sphincter |
| Somatic | Pudendal nerve (S2–S4) | Controls external sphincter (voluntary) |
🔁 IV. Voluntary vs Involuntary Control
- Infants and individuals with neurological damage lack voluntary control → reflex micturition occurs
- Toilet training teaches cortical control over external sphincter
- In adults, micturition is usually voluntary but can be overridden by high bladder pressure
🧾 V. Clinical Correlations
| Condition | Explanation |
|---|---|
| Urinary incontinence | Loss of bladder control (stress, urge, overflow types) |
| Neurogenic bladder | Nerve damage affecting bladder function |
| Urinary retention | Inability to empty bladder (e.g., prostate enlargement) |
| Overactive bladder | Frequent urge to urinate, detrusor overactivity |
| Spinal cord injury | Disrupts voluntary and reflex control pathways |
Micturition is a complex, reflex-driven yet voluntary process, tightly controlled by the autonomic and somatic nervous systems. Understanding its physiology is vital in diagnosing urinary disorders, managing catheterization, and promoting continence care in various healthcare settings.
🧠💧 Regulation of Renal Function
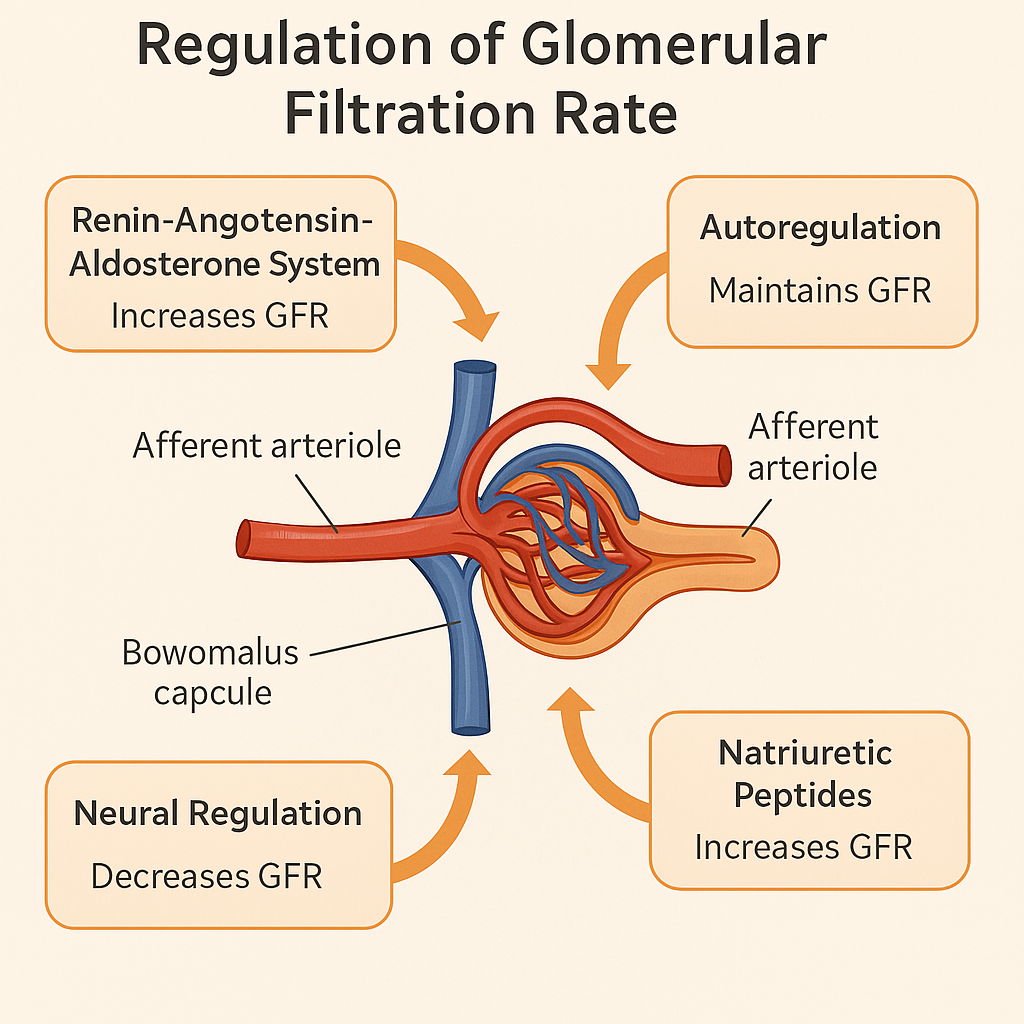
The kidneys are vital for maintaining homeostasis by regulating fluid balance, electrolyte levels, acid–base status, and blood pressure. They do this through filtration, reabsorption, secretion, and excretion of substances. These processes are finely controlled by intrinsic renal mechanisms and systemic hormonal and neural regulation.
🩺 I. Key Renal Processes Under Regulation
- Glomerular filtration – Initial filtering of blood at the glomerulus
- Tubular reabsorption – Selective movement of substances from tubules back to blood
- Tubular secretion – Transport of substances from blood into tubules
- Urine excretion – Final elimination of unneeded materials
These processes are tightly regulated to maintain internal balance.
🔬 II. Mechanisms Regulating Renal Function
🔹 1. Autoregulation (Intrinsic Renal Mechanism)
Maintains constant renal blood flow (RBF) and glomerular filtration rate (GFR) despite changes in systemic blood pressure (80–180 mmHg).
A. Myogenic Mechanism
- Afferent arteriole constricts when blood pressure rises → prevents over-filtration
- Dilates when pressure falls → maintains flow
B. Tubuloglomerular Feedback
- Macula densa cells in distal tubule sense NaCl levels
- If NaCl is high → afferent arteriole constricts → ↓ GFR
- If NaCl is low → afferent arteriole dilates → ↑ GFR
🔹 2. Hormonal Regulation
A. Renin–Angiotensin–Aldosterone System (RAAS)
- Activated by ↓ BP, ↓ Na⁺, or sympathetic stimulation
- Renin → converts angiotensinogen to angiotensin I → angiotensin II
- Angiotensin II:
- Constricts efferent arterioles → maintains GFR
- Stimulates aldosterone from adrenal cortex → Na⁺ and water reabsorption
- Increases thirst and ADH release
B. Antidiuretic Hormone (ADH)
- Released by posterior pituitary when osmolality ↑ or BP ↓
- Increases water reabsorption in the collecting ducts via aquaporins
- Concentrates urine and conserves water
C. Atrial Natriuretic Peptide (ANP)
- Released from atria in response to atrial stretch (volume overload)
- Inhibits Na⁺ and water reabsorption
- Dilates afferent arteriole → ↑ GFR
- Opposes RAAS
D. Parathyroid Hormone (PTH)
- Increases Ca²⁺ reabsorption and decreases phosphate reabsorption in renal tubules
🔹 3. Neural Regulation
- Sympathetic nervous system activation:
- Constricts renal vasculature → ↓ GFR during shock or stress
- Stimulates renin release
- Enhances Na⁺ reabsorption in proximal tubule
🔍 Useful in emergencies (e.g., hemorrhage) but harmful if prolonged (leads to ischemia).
🧬 III. Acid–Base Regulation by the Kidneys
- Reabsorb filtered bicarbonate (HCO₃⁻)
- Secrete hydrogen ions (H⁺) in proximal and distal tubules
- Generate new bicarbonate to buffer acids
🧾 IV. Summary of Key Regulators
| Mechanism | Function |
|---|---|
| Autoregulation | Maintains stable GFR |
| RAAS | Increases BP, Na⁺, water retention |
| ADH | Promotes water reabsorption in collecting ducts |
| ANP | Promotes Na⁺ excretion and reduces blood volume |
| PTH | Regulates calcium and phosphate reabsorption |
| Sympathetic nerves | Reduce GFR, activate RAAS |
| Tubuloglomerular feedback | Adjusts arteriole tone based on tubular flow |
🩺 Clinical Implications
- Hypertension: Often linked to overactive RAAS
- Heart failure: ADH and RAAS are elevated → fluid retention
- SIADH or diabetes insipidus: Disorders of ADH
- Renal artery stenosis: Activates RAAS → secondary hypertension
- Diuretics: Affect Na⁺, K⁺, and water reabsorption (act on specific nephron segments)
Renal function is regulated by a complex interplay of autonomic, hormonal, and local mechanisms that ensure stable internal conditions despite fluctuations in external inputs. These controls preserve blood pressure, fluid volume, electrolyte balance, and acid–base homeostasis, highlighting the kidney’s central role in systemic physiology.
💧 Renal System: Application and Implication in Nursing
The renal (urinary) system, consisting of the kidneys, ureters, bladder, and urethra, plays a central role in maintaining homeostasis through filtration, excretion, fluid-electrolyte balance, and blood pressure regulation. Nurses must understand renal physiology and pathology to accurately assess, monitor, and intervene in conditions affecting kidney function and urinary health.
🧠 I. Nursing Applications of Renal Physiology
1. Fluid and Electrolyte Balance Monitoring
- The kidneys regulate the volume and composition of body fluids.
- Nurses assess for signs of dehydration, fluid overload, and electrolyte imbalances (e.g., sodium, potassium, calcium).
- In patients on IV fluids or diuretics, strict input/output charting is essential.
2. Acid-Base Balance
- Kidneys excrete hydrogen ions and reabsorb bicarbonate, helping maintain blood pH.
- In metabolic acidosis or alkalosis, nurses monitor ABG values, respiratory compensation, and renal response.
3. Blood Pressure Regulation
- The renin-angiotensin-aldosterone system (RAAS), activated by the kidneys, maintains blood pressure.
- Nursing care includes monitoring BP trends, administering antihypertensives, and educating patients with renal hypertension.
4. Excretion of Waste Products
- The kidneys filter urea, creatinine, ammonia, and toxins.
- Serum creatinine, BUN, and eGFR are key markers nurses monitor for renal function assessment.
- Nurses play a role in early detection of renal insufficiency in high-risk patients (diabetics, elderly, etc.).
5. Erythropoiesis Regulation
- Kidneys secrete erythropoietin, which stimulates RBC production in the bone marrow.
- In chronic kidney disease (CKD), patients often develop anemia; nurses may administer erythropoiesis-stimulating agents (ESAs) and monitor hemoglobin levels.
💉 II. Nursing Implications in Renal Disorders
🏥 1. Acute and Chronic Kidney Disease
- Monitor urine output, edema, BP, and lab parameters (creatinine, GFR).
- Educate patients on renal-friendly diets (low sodium, potassium, protein in advanced stages).
- Prepare patients for dialysis, if necessary, and provide emotional support.
🚰 2. Urinary Tract Infections (UTIs)
- Common, especially in females, catheterized, or immobile patients.
- Nurses ensure hygiene, catheter care, and promote early detection (burning, frequency, cloudy urine).
- Encourage fluid intake and administer antibiotics as prescribed.
🩺 3. Renal Failure and Dialysis
- Monitor for uremia symptoms: confusion, pruritus, nausea.
- Assist with hemodialysis or peritoneal dialysis care, including:
- Access site monitoring
- Infection control
- Fluid restrictions and dietary education
💊 4. Drug Administration Considerations
- Many drugs are excreted by the kidneys.
- Dosages may need adjustment in renal impairment (e.g., aminoglycosides, digoxin).
- Nurses must calculate renal-safe doses and monitor for drug toxicity.
🛏 5. Urinary Incontinence and Retention
- Assess bladder distension, residual volume (using a bladder scanner).
- Implement bladder training, pelvic floor exercises, or catheterization as needed.
- Provide psychosocial support to manage embarrassment and isolation.
📚 III. Key Nursing Responsibilities Related to the Renal System
- Assess: Urine output, color, clarity, odor, lab values, weight changes, vital signs
- Educate: Fluid restrictions, dietary modifications, infection prevention
- Intervene: Administer diuretics, antihypertensives, ESAs; monitor electrolytes
- Prevent: Catheter-associated UTIs, renal damage from nephrotoxic drugs
- Support: Emotional and psychological needs in patients with CKD, dialysis, or renal transplant
🧾 IV. Examples of Nursing Scenarios Involving Renal System
| Clinical Scenario | Nursing Focus |
|---|---|
| Post-operative patient with Foley catheter | Prevent CAUTI, ensure catheter patency, monitor output |
| Patient on furosemide | Monitor potassium levels, BP, signs of dehydration |
| Elderly with urinary incontinence | Skin care, fall prevention, toileting schedule |
| Diabetic patient with early CKD | Educate on protein intake, monitor microalbuminuria |
| ESRD patient on hemodialysis | Monitor for hypotension, access site bleeding/infection |