Physio-Unit-5-B.sc-Blood
🩸 Composition of Blood

Blood is a specialized connective tissue that plays a central role in transport, regulation, and protection within the body. It circulates through a closed system of blood vessels and is essential for maintaining homeostasis.
🧠
- Total blood volume in an average adult: ~5–6 liters
- Blood consists of two main components:
- Plasma (liquid component) – ~55%
- Formed elements (cellular components) – ~45%
💧 II. Plasma (55% of blood volume)
Plasma is the straw-colored fluid that remains after removal of blood cells. It is ~92% water and serves as the transport medium for nutrients, hormones, and waste.
🧪 Composition of Plasma:
| Component | Percentage/Details | Function |
|---|---|---|
| Water (92%) | Solvent medium | Maintains volume and transports substances |
| Plasma proteins (7%) | Albumin, globulins, fibrinogen | Maintain osmotic pressure, immunity, clotting |
| Electrolytes | Na⁺, K⁺, Ca²⁺, Cl⁻, HCO₃⁻ | Acid-base balance, nerve/muscle function |
| Nutrients | Glucose, amino acids, lipids | Energy supply and tissue repair |
| Gases | O₂, CO₂, N₂ | Cellular respiration and waste elimination |
| Waste products | Urea, creatinine, bilirubin | Excreted by kidneys or liver |
| Hormones and enzymes | Various | Regulation of physiological processes |
🧬 III. Formed Elements (45% of blood volume)
These are the cellular components suspended in plasma, produced primarily in the red bone marrow.
1. Red Blood Cells (RBCs) / Erythrocytes
- Most abundant formed element (~4.5–6 million/µL)
- Structure: Biconcave, anucleate, flexible
- Function: Transport oxygen (via hemoglobin) and carbon dioxide
- Lifespan: ~120 days
- Normal hematocrit: ~40–45%
2. White Blood Cells (WBCs) / Leukocytes
- ~4,000–11,000/µL
- Function: Defense and immunity
- Types (divided into granulocytes and agranulocytes):
a. Granulocytes
| Type | % of WBCs | Function |
|---|---|---|
| Neutrophils | 60–70% | Phagocytosis of bacteria |
| Eosinophils | 2–4% | Combat parasites, allergic response |
| Basophils | <1% | Release histamine in inflammation |
b. Agranulocytes
| Type | % of WBCs | Function |
|---|---|---|
| Lymphocytes | 20–25% | B and T cells – immunity |
| Monocytes | 3–8% | Phagocytosis, become macrophages |
3. Platelets / Thrombocytes
- 150,000–400,000/µL
- Cell fragments from megakaryocytes
- Function: Initiate clot formation to prevent bleeding
- Lifespan: ~7–10 days
🔁 IV. Summary Table: Composition of Blood
| Component | % of Total Volume | Key Function |
|---|---|---|
| Plasma | ~55% | Transport, pH balance, immunity, clotting |
| RBCs | ~44% | Oxygen and CO₂ transport |
| WBCs | <1% | Defense against pathogens |
| Platelets | <1% | Hemostasis (clot formation) |
⚠️ Clinical Relevance
| Condition | Blood Composition Change |
|---|---|
| Anemia | ↓ RBC count/hemoglobin |
| Leukocytosis | ↑ WBC count (infection, leukemia) |
| Thrombocytopenia | ↓ Platelets (bleeding risk) |
| Dehydration | ↑ Hematocrit due to plasma volume loss |
| Hyperproteinemia | ↑ Plasma proteins (e.g., in multiple myeloma) |
| Hypoproteinemia | ↓ Albumin (malnutrition, liver disease) |
✅
Blood is a complex, multifunctional fluid made of plasma and formed elements. Its composition reflects and affects nearly every aspect of human physiology—transport, immunity, clotting, and pH balance—making it central to both health assessment and disease diagnosis.
🩸 Functions of Blood

Blood is a life-sustaining fluid tissue that circulates through the cardiovascular system, delivering substances essential for cellular functions and removing waste products. Its dynamic composition supports multiple physiological systems—including respiratory, immune, excretory, regulatory, and endocrine functions.
Blood performs three main categories of functions:
- Transport
- Regulation
- Protection
🧠 I. Transport Functions of Blood
Blood serves as the primary transport medium in the body.
1. Oxygen Transport
- Red blood cells (RBCs) contain hemoglobin, which binds and carries oxygen (O₂) from the lungs to tissues.
2. Carbon Dioxide Removal
- Carries CO₂ from tissues to the lungs for exhalation.
3. Nutrient Distribution
- Delivers absorbed glucose, amino acids, lipids, vitamins, and minerals from the digestive tract to body cells.
4. Hormone Circulation
- Transports hormones from endocrine glands to target organs for physiological regulation.
5. Waste Elimination
- Transports metabolic waste products (urea, creatinine, bilirubin) to the kidneys, liver, lungs, and skin for excretion.
🔁 II. Regulatory Functions of Blood
Blood plays a key role in homeostasis by regulating internal environmental conditions.
1. pH Balance
- Acts as a buffer system (mainly bicarbonate) to maintain physiological pH ~7.35–7.45.
2. Body Temperature Regulation
- Redistributes heat through vasodilation (heat loss) or vasoconstriction (heat conservation).
- Maintains core body temperature (~37°C).
3. Fluid and Electrolyte Balance
- Plasma proteins (e.g., albumin) maintain colloid osmotic pressure, regulating water exchange between blood and tissues.
4. Blood Volume Regulation
- Hormonal systems (e.g., ADH, aldosterone) influence blood volume by regulating water retention and sodium balance.
🛡️ III. Protective Functions of Blood
The blood helps defend the body against pathogens, injury, and bleeding.
1. Immunity
- White blood cells (WBCs) identify, destroy, and remember pathogens.
- Neutrophils: Phagocytosis of bacteria
- Lymphocytes: Antibody production and immune memory
- Monocytes: Transform into macrophages
- Blood carries antibodies and complement proteins aiding in immune response.
2. Hemostasis (Prevention of Blood Loss)
- Platelets, fibrinogen, and clotting factors initiate and stabilize clots at injury sites.
3. Detoxification
- Blood transports toxins to liver and kidneys for neutralization or excretion.
📊 IV. Blood Functions
| Category | Specific Function |
|---|---|
| Transport | O₂, CO₂, nutrients, hormones, waste |
| Regulation | pH, temperature, osmotic balance, blood volume |
| Protection | Immune defense, clotting, wound healing, detox |
🧬 V. Clinical Relevance of Blood Functions
| Condition | Impaired Function |
|---|---|
| Anemia | ↓ Oxygen-carrying capacity |
| Leukopenia/Leukocytosis | Immune suppression / hyperactivity |
| Dehydration | ↓ Plasma volume → hypotension |
| Liver failure | ↓ Clotting proteins → bleeding risk |
| Sepsis | Disrupted regulation → shock, clotting imbalance |
✅
Blood is not just a transport medium—it is a multifunctional connective tissue that plays crucial roles in sustaining life, maintaining balance, and defending against harm. For nurses and clinicians, recognizing alterations in these functions is vital for early diagnosis, treatment, and care planning in both acute and chronic illnesses.
🩸 Physical Characteristics of Blood:-

Blood is a specialized connective tissue with unique physical properties that allow it to circulate efficiently throughout the vascular system. Its color, volume, viscosity, temperature, and pH contribute to its ability to perform vital transport, regulatory, and protective functions.
🧪 I. Color of Blood
- Oxygenated blood: Bright red
- Due to oxyhemoglobin formed when oxygen binds to hemoglobin in arterial blood.
- Deoxygenated blood: Dark red to bluish-red
- Due to reduced hemoglobin in venous blood.
- Venous blood appears bluish through the skin because of light refraction, not its actual color.
🧠 Color changes are clinically significant in assessing hypoxia, cyanosis, or carbon monoxide poisoning.
💉 II. Volume of Blood
- Total blood volume in an average adult:
- Male: ~5–6 liters
- Female: ~4–5 liters
- Varies with body size, age, gender, and hydration status.
- ~8% of total body weight.
🩺 Blood volume is crucial in managing fluid therapy, blood transfusions, and shock.
🧈 III. Viscosity (Thickness)
- Blood is 4–5 times more viscous than water due to:
- High content of cells (mainly RBCs)
- Presence of plasma proteins (e.g., fibrinogen, globulins)
- Increased viscosity = greater resistance to flow.
🔍 Clinical Relevance:
- High viscosity: Polycythemia, dehydration → ↑ cardiac workload.
- Low viscosity: Anemia → ↓ oxygen-carrying capacity.
🌡️ IV. Temperature
- Blood temperature is slightly higher than body temperature:
- Normal: ~38°C (100.4°F)
- Blood helps distribute heat produced by metabolism throughout the body.
🧠 Useful in thermoregulation and fever response.
⚖️ V. pH of Blood
- Slightly alkaline:
- Normal range: 7.35–7.45
- Maintained by buffer systems (bicarbonate, hemoglobin, phosphate), respiratory regulation, and renal function.
⚠️ Deviations:
- Acidosis: pH < 7.35 → CNS depression, coma
- Alkalosis: pH > 7.45 → Muscle excitability, arrhythmias
🧬 VI. Specific Gravity
- Blood: 1.050–1.060
- Plasma: 1.025–1.030
- RBCs: 1.090
- Influenced by cell concentration and plasma proteins.
💨 VII. Odor and Taste
- Blood has a metallic taste due to iron.
- Odor is due to volatile substances like urea and organic acids.
(Not typically used in diagnosis but relevant to understanding waste accumulation in renal failure or sepsis.)
🔁 VIII. Osmotic Pressure
- Maintained by plasma proteins, especially albumin.
- Helps retain water within the vascular system and prevents excessive fluid leakage into tissues.
🧾Physical Characteristics of Blood
| Characteristic | Normal Value/Description | Clinical Significance |
|---|---|---|
| Color | Bright red (oxygenated), dark red (deoxygenated) | Reflects oxygen saturation |
| Volume | 4–6 L (avg adult) | Guides fluid replacement, transfusion |
| Viscosity | 4–5× thicker than water | Affects blood flow and cardiac workload |
| Temperature | ~38°C (100.4°F) | Thermoregulation |
| pH | 7.35–7.45 (slightly alkaline) | Buffer function, metabolic and respiratory balance |
| Specific gravity | 1.050–1.060 | Relates to cell/plasma content |
| Osmotic pressure | ~25 mmHg (colloid pressure) | Maintains fluid balance in capillaries |
✅
The physical properties of blood are essential for its role in circulation, oxygen delivery, waste removal, and regulation of body conditions. These characteristics form the foundation for interpreting clinical symptoms, performing diagnostic tests, and providing targeted care in various health conditions.
🧬 Formation of Blood Cells (Hematopoiesis)
Hematopoiesis (or hemopoiesis) is the physiological process by which the body produces blood cells. It occurs continuously throughout life to replenish cells that are used, aged, or lost due to bleeding or disease.
All blood cells originate from pluripotent hematopoietic stem cells in the red bone marrow through a tightly regulated process involving growth factors, cytokines, and cell differentiation.
🧠 I. Sites of Hematopoiesis
| Life Stage | Primary Sites |
|---|---|
| Embryonic (first 2 months) | Yolk sac |
| Fetal (2–7 months) | Liver and spleen |
| Late fetal and after birth | Red bone marrow (sternum, ribs, pelvis, vertebrae, skull, proximal long bones) |
In adults, flat bones are the major hematopoietic sites.
🧪 II. Types of Blood Cells Formed
All blood cells arise from hematopoietic stem cells (HSCs), which differentiate into:
1. Myeloid Stem Cells → give rise to:
- Erythrocytes (RBCs)
- Megakaryocytes → Platelets
- Granulocytes (Neutrophils, Eosinophils, Basophils)
- Monocytes
2. Lymphoid Stem Cells → give rise to:
- T lymphocytes
- B lymphocytes
- Natural killer (NK) cells
🔁 III. Hematopoietic Process: Lineage Differentiation
🔴 1. Erythropoiesis (RBC Formation)
- Origin: Myeloid stem cell
- Key hormone: Erythropoietin (EPO) from kidneys (in response to hypoxia)
- Stages:
- Proerythroblast
- Erythroblast → Normoblast
- Reticulocyte (immature RBC)
- Mature erythrocyte
- Time: ~5–7 days
- Nutritional requirements: Iron, vitamin B12, folic acid, protein
🟣 2. Thrombopoiesis (Platelet Formation)
- Origin: Myeloid stem cell
- Key hormone: Thrombopoietin (TPO) from liver
- Megakaryoblast → Megakaryocyte → Fragments into ~1,000–3,000 platelets
- Lifespan: ~7–10 days
⚪ 3. Leukopoiesis (WBC Formation)
a. Granulopoiesis (Neutrophils, Eosinophils, Basophils)
- Myeloblast → Promyelocyte → Myelocyte → Metamyelocyte → Band cell → Mature granulocyte
- Stimulated by: G-CSF, GM-CSF
b. Monopoiesis (Monocytes)
- Monoblast → Promonocyte → Monocyte → Macrophage (in tissue)
c. Lymphopoiesis (Lymphocytes)
- Lymphoid stem cell → B or T cell precursor
- B cells mature in bone marrow
- T cells mature in thymus
Lymphocytes are key players in adaptive immunity.
📦 IV. Blood Cell Formation
| Cell Type | Origin | Key Hormone | Primary Function |
|---|---|---|---|
| RBCs | Myeloid stem cell | Erythropoietin | O₂ and CO₂ transport |
| Platelets | Myeloid stem cell | Thrombopoietin | Blood clotting |
| Neutrophils | Myeloid stem cell | G-CSF | Phagocytosis of bacteria |
| Eosinophils | Myeloid stem cell | GM-CSF | Allergy & parasite defense |
| Basophils | Myeloid stem cell | GM-CSF | Release histamine in inflammation |
| Monocytes | Myeloid stem cell | M-CSF | Become macrophages; phagocytosis |
| B Lymphocytes | Lymphoid stem cell | IL-7 | Antibody production |
| T Lymphocytes | Lymphoid stem cell | IL-2, IL-7 | Cell-mediated immunity |
| NK Cells | Lymphoid stem cell | IL-15 | Non-specific immune response |
🧬 V. Regulation of Hematopoiesis
- Controlled by growth factors, cytokines, and local bone marrow microenvironment
- Regulated in response to:
- Tissue hypoxia → increases RBC production
- Infection → increases WBC production
- Bleeding or clotting → increases platelet production
🩺 VI. Clinical Relevance
| Condition | Effect on Hematopoiesis |
|---|---|
| Anemia | Decreased erythropoiesis or nutrient deficiency |
| Leukemia | Uncontrolled proliferation of abnormal WBCs |
| Aplastic anemia | Bone marrow failure (↓ all cell lines) |
| Polycythemia vera | Excess RBC production → ↑ viscosity, clot risk |
| Chemotherapy | Suppresses bone marrow → pancytopenia |
| Bone marrow transplant | Replaces defective hematopoietic stem cells |
✅
Blood cell formation (hematopoiesis) is a complex, finely regulated process vital for:
- Oxygen transport
- Immunity
- Hemostasis
Understanding its mechanisms enables healthcare professionals to interpret CBC results, manage blood disorders, and support patients undergoing therapies that affect bone marrow function.
🔴 Erythropoiesis – Functions of RBCs and RBC Life Cycle

🧬 I. What is Erythropoiesis?
Erythropoiesis is the process of red blood cell (erythrocyte) formation. It occurs in the red bone marrow and is stimulated by hypoxia (low oxygen levels in tissues), which triggers the release of erythropoietin, a hormone produced mainly by the kidneys.
🔹 Site of Erythropoiesis:
- In fetus: Liver, spleen
- In children and adults: Red bone marrow of flat bones (sternum, ribs, vertebrae, pelvis) and long bone epiphyses
🧠 II. Stages of Erythropoiesis
Erythropoiesis involves the transformation of a pluripotent stem cell into a mature RBC over ~5–7 days:
- Hematopoietic Stem Cell (HSC)
- Myeloid Stem Cell
- Proerythroblast – Large, nucleated
- Basophilic Erythroblast – Begins hemoglobin synthesis
- Polychromatic Erythroblast – More hemoglobin, less basophilia
- Orthochromatic Erythroblast (Normoblast) – Nucleus becomes pyknotic
- Reticulocyte – Nucleus extruded, enters circulation
- Mature Erythrocyte (RBC) – Fully functional, biconcave, anucleate
🧪 Reticulocyte count (~1–2%) reflects bone marrow activity.
💉 III. Hormonal and Nutritional Requirements
| Factor | Role |
|---|---|
| Erythropoietin | Stimulates proliferation & differentiation |
| Iron | Required for hemoglobin synthesis |
| Vitamin B₁₂ & Folate | Needed for DNA synthesis in erythroblasts |
| Amino acids & Copper | Support globin and enzyme production |
🩸 IV. Structure and Functions of RBCs
🔶 Structure:
- Biconcave discs, ~7.5 μm diameter
- No nucleus or organelles
- Flexible membrane (to pass through capillaries)
- Contains ~280 million hemoglobin molecules per cell
🔷 Functions of RBCs:
| Function | Explanation |
|---|---|
| Oxygen transport | Hemoglobin binds O₂ in lungs and releases it in tissues |
| Carbon dioxide transport | Carries CO₂ from tissues to lungs (as carbaminohemoglobin or HCO₃⁻) |
| Buffering pH | Hemoglobin acts as a buffer by binding H⁺ and maintaining blood pH |
| Maintaining blood viscosity | Contributes to blood flow dynamics and pressure |
🔄 V. Life Cycle of RBCs
1. Production (Erythropoiesis)
- Occurs in red bone marrow
- Takes about 5–7 days
2. Circulation
- Mature RBCs circulate for about 120 days
- Travel ~250 km in their lifespan!
3. Senescence (Aging)
- RBCs become less flexible and are removed by macrophages (mainly in the spleen, liver, and bone marrow)
4. Destruction and Recycling
- Hemoglobin breakdown:
- Globin chains → amino acids → reused
- Iron → bound to transferrin → reused in marrow
- Heme → biliverdin → bilirubin → excreted in bile
🩺 VI. Clinical Relevance
| Condition | Effect on Erythropoiesis / RBCs |
|---|---|
| Anemia | ↓ RBCs or hemoglobin → fatigue, pallor |
| Polycythemia vera | Excess RBCs → ↑ blood viscosity, clot risk |
| Iron-deficiency anemia | Poor hemoglobin synthesis |
| Vitamin B12/Folate deficiency | Impaired DNA synthesis → megaloblastic anemia |
| Chronic kidney disease | ↓ erythropoietin production → anemia |
| Hemolytic anemia | Premature RBC destruction |
🧾 Summary Chart
| Stage | Key Feature | Time |
|---|---|---|
| Proerythroblast | Large, nucleated cell | Day 1 |
| Erythroblast | Hemoglobin starts to form | Days 2–3 |
| Normoblast | Nucleus condenses and is expelled | Day 4 |
| Reticulocyte | Enters blood, still has RNA | Day 5–6 |
| Mature RBC | Fully functional, anucleate | Day 7 |
| Lifespan | Circulates, performs functions | ~120 days |
✅
Erythropoiesis is the vital process of red blood cell production, essential for oxygen delivery and acid-base regulation. The mature RBC, though simple in structure, plays a critical role in sustaining life and is a key indicator of many systemic health conditions.
⚪ White Blood Cells (WBCs) – Types, Functions, and Academic Overview

White Blood Cells (WBCs), also known as leukocytes, are the body’s primary defense cells, protecting against infections, foreign bodies, allergens, and abnormal cells (e.g., cancerous cells). Unlike RBCs, WBCs are nucleated, colorless, and capable of movement through tissue spaces.
🧠 I. General Characteristics of WBCs
- Count in blood: 4,000–11,000 cells/μL
- Lifespan: A few hours to several days (some memory cells last for years)
- Location: Found in bloodstream and tissues (via diapedesis)
- Produced in bone marrow (and lymphatic tissues for lymphocytes)
🧪 II. Classification of WBCs
WBCs are classified into two major groups based on the presence or absence of cytoplasmic granules:
🔹 A. Granulocytes (with visible granules; multilobed nuclei)
| Type | Abundance in Blood | Key Functions |
|---|---|---|
| Neutrophils | 60–70% | First responders; phagocytose bacteria, fungi |
| Eosinophils | 2–4% | Attack parasites; modulate allergic response |
| Basophils | <1% | Release histamine in allergies/inflammation |
🔹 B. Agranulocytes (no visible granules; rounded or indented nuclei)
| Type | Abundance in Blood | Key Functions |
|---|---|---|
| Lymphocytes | 20–25% | Adaptive immunity (B and T cells) |
| Monocytes | 3–8% | Differentiate into macrophages; phagocytosis |
🧬 III. Detailed Functions of Each WBC Type
1. Neutrophils
- Most abundant WBC
- Function:
- Phagocytosis of bacteria and debris
- Release of lysozymes, oxidants, and defensins
- Lifespan: ~6 hours in circulation; longer in tissues
- Elevated in bacterial infections, inflammation, and stress
2. Eosinophils
- Contain acidic granules with enzymes (e.g., peroxidase, major basic protein)
- Function:
- Kill parasitic worms
- Modulate allergic reactions by deactivating histamine
- Elevated in parasitic infections, allergic disorders (e.g., asthma)
3. Basophils
- Least abundant WBCs
- Granules contain histamine, heparin, and serotonin
- Function:
- Involved in immediate hypersensitivity reactions (e.g., anaphylaxis)
- Enhance inflammatory response
- Related to mast cells in tissues
4. Lymphocytes
- Exist as:
- B cells: Produce antibodies (humoral immunity)
- T cells: Attack virus-infected, cancer cells (cell-mediated immunity)
- NK (Natural Killer) cells: Destroy abnormal cells nonspecifically
- Lifespan: Days to years (memory cells)
- Elevated in viral infections, some chronic infections
5. Monocytes
- Largest WBCs; kidney-shaped nucleus
- Migrate into tissues and become macrophages
- Function:
- Phagocytosis of pathogens and debris
- Antigen presentation to lymphocytes
- Secrete cytokines to regulate immune response
- Elevated in chronic infections like TB, malaria
📦 IV. Summary Table of WBC Types and Functions
| Type | Appearance | Key Role |
|---|---|---|
| Neutrophils | Multi-lobed nucleus, pale granules | Phagocytosis of bacteria |
| Eosinophils | Bi-lobed nucleus, red granules | Parasite killing, allergy moderation |
| Basophils | Dark purple granules | Histamine release, allergic reactions |
| Lymphocytes | Large round nucleus, thin cytoplasm | Adaptive immunity (B, T, NK cells) |
| Monocytes | Kidney-shaped nucleus | Phagocytosis, macrophage precursor |
🧾 V. Clinical Relevance
| Condition | WBC Change |
|---|---|
| Bacterial infection | ↑ Neutrophils (Neutrophilia) |
| Viral infection | ↑ Lymphocytes (Lymphocytosis) |
| Parasitic infection | ↑ Eosinophils (Eosinophilia) |
| Allergic reaction | ↑ Basophils and Eosinophils |
| Chronic inflammation | ↑ Monocytes (Monocytosis) |
| Leukopenia | ↓ Total WBCs (e.g., chemotherapy) |
| Leukemia | Uncontrolled WBC production (immature) |
✅
White blood cells are key players in immune surveillance, defense, and tissue repair. Each type has a specialized function, and their levels are crucial indicators in infection, inflammation, immune disorders, and hematologic diseases.
Understanding their types and roles equips healthcare professionals to interpret CBCs, manage immunocompromised patients, and respond to infectious or allergic emergencies.
🩸 Platelets – Function and Production (Thrombopoiesis)

Platelets, or thrombocytes, are cell fragments that play a crucial role in blood clotting (hemostasis) and wound healing. They are not true cells but are derived from megakaryocytes in the bone marrow.
🧠 I. Characteristics of Platelets
| Feature | Details |
|---|---|
| Shape | Small, disc-shaped fragments |
| Size | ~2–3 µm in diameter |
| Count (normal) | 150,000–400,000/µL of blood |
| Lifespan | 7–10 days in circulation |
| Removal | Cleared by spleen and liver macrophages |
Platelets lack a nucleus, but contain granules with important substances for clotting and tissue repair.
🧬 II. Production of Platelets – Thrombopoiesis
Thrombopoiesis is the process of platelet formation and maturation in the red bone marrow.
🔹 Steps of Thrombopoiesis:
- Hematopoietic stem cell (HSC)
- Myeloid stem cell
- Megakaryoblast
- Promegakaryocyte
- Megakaryocyte – large cell (~50–100 µm), polyploid nucleus
- Platelet formation – cytoplasmic fragments bud off into the bloodstream
🔸 Key Regulator: Thrombopoietin (TPO)
- A glycoprotein hormone mainly produced by the liver
- Stimulates megakaryocyte proliferation and maturation
📦 III. Functions of Platelets
1. Primary Hemostasis (Platelet Plug Formation)
- Adhesion: Platelets adhere to exposed collagen at a vessel injury site (via von Willebrand factor).
- Activation: Platelets change shape, release granules (e.g., ADP, thromboxane A2) to recruit more platelets.
- Aggregation: Platelets stick to each other forming a platelet plug.
2. Secondary Hemostasis (Coagulation Cascade Support)
- Provide phospholipid surface for activation of clotting factors.
- Support formation of fibrin mesh to stabilize the clot.
3. Wound Healing
- Release growth factors (e.g., platelet-derived growth factor – PDGF) that:
- Stimulate fibroblast proliferation
- Promote angiogenesis
- Aid in tissue regeneration
4. Vascular Integrity
- Maintain endothelial function in microvessels and prevent spontaneous leakage.
🩺 IV. Clinical Relevance of Platelet Function
| Condition | Effect |
|---|---|
| Thrombocytopenia | Platelet count <150,000/µL → bleeding risk |
| Thrombocytosis | Platelet count >400,000/µL → clot risk |
| Aspirin/NSAIDs use | Inhibits platelet aggregation |
| Leukemia or chemotherapy | Suppressed marrow → low platelet production |
| ITP (Immune Thrombocytopenia) | Autoimmune destruction of platelets |
🧪 Platelet function tests (e.g., bleeding time, platelet aggregation) help assess clotting ability.
🧾 Summary Table
| Feature | Detail |
|---|---|
| Origin | Megakaryocytes in bone marrow |
| Hormone Stimulus | Thrombopoietin (TPO) |
| Normal Count | 150,000–400,000/μL |
| Lifespan | 7–10 days |
| Function | Hemostasis, clot formation, healing |
| Clinical Disorders | Thrombocytopenia, thrombocytosis |
✅
Platelets are indispensable for hemostasis, enabling the body to quickly respond to vascular injury and initiate clotting. They also support wound healing and help maintain vascular integrity. Their production (thrombopoiesis) is tightly regulated, and dysfunction can lead to life-threatening bleeding or thrombosis.
🩸 Clotting Mechanism of Blood

Blood clotting (also known as coagulation) is a complex physiological process that prevents excessive blood loss following vascular injury. It involves platelets, clotting factors, vascular endothelium, and fibrin formation.
🧬 I. Mechanism of Blood Clotting
The clotting process occurs in three phases:
🧪 Phase 1: Formation of Prothrombin Activator (Initiation Phase)
This occurs through two pathways, both leading to activation of Factor X:
🔹 A. Intrinsic Pathway
- Activated by trauma inside the blood vessel (e.g., collagen exposure)
- Involves Factors XII, XI, IX, VIII
- Slower but more sustained
🔹 B. Extrinsic Pathway
- Activated by external trauma that exposes tissue factor (TF or Factor III)
- Involves Factor VII
- Rapid response
✅ Both pathways converge to activate Factor X, beginning the common pathway.
🧪 Phase 2: Conversion of Prothrombin to Thrombin
- Prothrombin (Factor II) → Thrombin
- Requires calcium ions (Ca²⁺) and phospholipids from platelets
🧪 Phase 3: Formation of Fibrin Mesh
- Thrombin converts fibrinogen (Factor I) → Fibrin
- Fibrin strands form a mesh that stabilizes the platelet plug
- Factor XIII (fibrin-stabilizing factor) cross-links fibrin to strengthen the clot
📦 II. Summary Table of Coagulation Pathways
| Pathway | Trigger | Key Factors Involved |
|---|---|---|
| Intrinsic | Contact with damaged endothelium | XII, XI, IX, VIII, X, V, II, I |
| Extrinsic | Tissue trauma (TF release) | VII, X, V, II, I |
| Common Pathway | Activation of Factor X | X → Prothrombin → Thrombin → Fibrinogen → Fibrin |
🧠 III. Clotting Time (CT)
🔹 Definition:
Time taken for blood to form a clot in vitro after being exposed to air.
- Normal range: 5–11 minutes
- Measured using capillary tube method or Lee-White method
- Evaluates the intrinsic pathway
🩺 Prolonged clotting time seen in hemophilia, severe liver disease, anticoagulant therapy
💉 IV. Bleeding Time (BT)
🔹 Definition:
Time taken for bleeding to stop from a small skin puncture.
- Normal range: 1–6 minutes
- Measured using Duke’s method or Ivy’s method
- Reflects platelet function and vascular integrity
🩺 Prolonged bleeding time in thrombocytopenia, von Willebrand disease, aspirin use
🔬 V. Partial Thromboplastin Time (PTT / aPTT)
🔹 Definition:
Time taken for plasma to clot after adding calcium, phospholipid, and an activator.
- Normal range: 25–40 seconds
- Measures intrinsic and common coagulation pathways
- Used to monitor heparin therapy
🩺 Prolonged PTT in:
- Hemophilia A (Factor VIII deficiency)
- Hemophilia B (Factor IX deficiency)
- Heparin overdose
- Lupus anticoagulant
🧾 VI. Comparison Table: BT vs CT vs PTT
| Test | Measures | Normal Range | Used For |
|---|---|---|---|
| Bleeding Time | Platelet function & capillary integrity | 1–6 minutes | von Willebrand disease, aspirin use |
| Clotting Time | Coagulation time in whole blood | 5–11 minutes | Hemophilia, liver disease |
| PTT / aPTT | Intrinsic & common pathway factors | 25–40 seconds | Monitor heparin, screen hemophilia |
🧪 VII. Clot Retraction and Fibrinolysis
After clot formation:
- Platelets contract → clot retraction
- Later, plasminogen is activated to plasmin
- Plasmin digests fibrin → fibrinolysis (clot breakdown)
🩺 VIII. Clinical Relevance
| Condition | Coagulation Profile |
|---|---|
| Hemophilia A/B | ↑ PTT, normal BT & PT |
| Von Willebrand disease | ↑ BT, possibly ↑ PTT |
| Liver disease | ↑ CT, PT, PTT due to impaired clotting factor synthesis |
| Heparin therapy | ↑ aPTT |
| Warfarin therapy | ↑ PT, normal or mildly ↑ aPTT |
✅
The clotting mechanism is a finely regulated cascade involving platelets, clotting factors, and fibrin, ensuring that bleeding stops while maintaining normal circulation. Laboratory tests like BT, CT, and PTT help in diagnosing coagulation disorders, monitoring therapies, and guiding patient management.
🩸 Hemostasis – Role of Vasoconstriction (Vascular Spasm)
Vasoconstriction, also called vascular spasm, is the first immediate response in the process of hemostasis—the body’s way of stopping blood loss after injury to a blood vessel. It is crucial for minimizing blood flow to the damaged area and giving time for platelet plug formation and coagulation to occur.
🧠 Mechanism of Vasoconstriction
When a blood vessel is injured, the smooth muscle in the vessel wall contracts. This constriction:
- Narrows the vessel lumen
- Reduces blood flow
- Minimizes blood loss at the injury site
🔍 Triggers of Vasoconstriction in Hemostasis
- Local myogenic response
- Direct mechanical injury to vascular smooth muscle triggers an automatic contraction.
- Release of vasoactive substances from platelets:
- Serotonin
- Thromboxane A₂ (TXA₂)
- Both are potent vasoconstrictors released from platelet granules.
- Endothelial-derived factors:
- Endothelin-1, secreted by damaged endothelium, promotes smooth muscle contraction.
- Neural reflexes:
- Pain and trauma activate sympathetic vasoconstrictor nerves.
📌 Duration and Importance
- Vasoconstriction is transient (lasting minutes to hours) but critical.
- It slows down blood loss to allow platelets and coagulation factors to act effectively.
- In small vessels, vasoconstriction alone may be enough to stop bleeding temporarily.
🩺 Clinical Relevance
- Poor vasoconstriction (e.g., in capillary fragility, platelet disorders) can lead to prolonged bleeding.
- Some vasoconstrictor drugs (like epinephrine) are used during surgeries or in bleeding control to reduce local blood flow.
- Excessive vasoconstriction can cause ischemia if not balanced by proper clot resolution (fibrinolysis).
✅
| Aspect | Details |
|---|---|
| What is it? | Constriction of injured blood vessel |
| When does it occur? | Immediately after vascular injury |
| Why is it important? | Reduces blood loss and prepares for next steps |
| Mediators involved | Serotonin, Thromboxane A₂, Endothelin |
| Clinical importance | Aids in surgical bleeding control, trauma care |
🟡 Platelet Plug Formation in Hemostasis
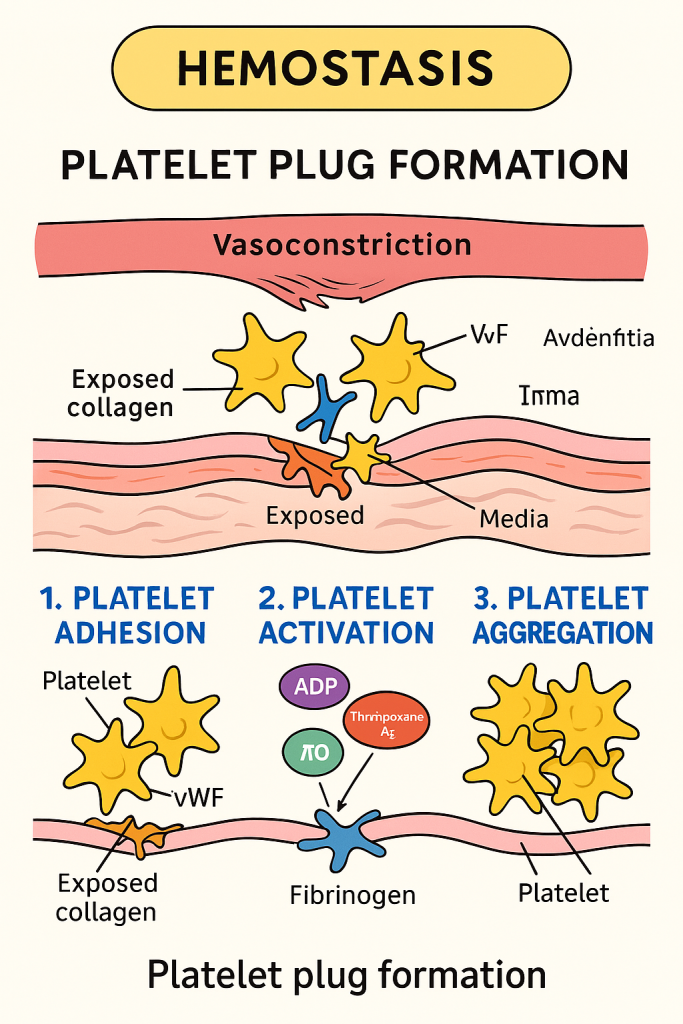
Platelet plug formation is the second critical step in the hemostatic process, following vascular spasm (vasoconstriction). It constitutes primary hemostasis, where platelets adhere, activate, and aggregate at the site of vascular injury to form a temporary plug that seals small vessel injuries.
🧠 Why is Platelet Plug Formation Important?
- Prevents blood loss from microvascular injuries
- Initiates coagulation cascade for fibrin clot formation
- Provides a surface for clotting factors to bind and activate
🩸 Stages of Platelet Plug Formation
🔹 1. Platelet Adhesion
- Platelets adhere to exposed subendothelial collagen fibers at the injury site.
- von Willebrand Factor (vWF) acts as a bridge between collagen and platelet receptors (GPIb).
- Occurs within seconds after endothelial injury.
🔹 2. Platelet Activation
Once adhered, platelets undergo several changes:
- Change in shape (become spiky and sticky)
- Release of granule contents:
- ADP – promotes aggregation
- Thromboxane A₂ (TXA₂) – vasoconstriction and platelet recruitment
- Serotonin – enhances vasoconstriction
- Expression of GPIIb/IIIa receptors, which are essential for aggregation
🔹 3. Platelet Aggregation
- Fibrinogen binds to the activated GPIIb/IIIa receptors on adjacent platelets
- Forms bridges between platelets → platelet plug
- The plug is temporary and needs to be stabilized by fibrin (secondary hemostasis)
📊 Key Molecules Involved
| Substance | Source | Function |
|---|---|---|
| von Willebrand factor (vWF) | Damaged endothelium, platelets | Platelet adhesion to collagen |
| ADP | Platelet granules | Platelet activation and aggregation |
| Thromboxane A₂ | Platelets | Vasoconstriction and platelet recruitment |
| Serotonin | Platelets | Promotes vasoconstriction |
| Fibrinogen | Plasma | Forms bridges between platelets |
🧪 Platelet Plug vs Fibrin Clot
| Platelet Plug (Primary Hemostasis) | Fibrin Clot (Secondary Hemostasis) |
|---|---|
| Formed by platelets only | Stabilized by fibrin |
| Quick, temporary | Delayed, long-lasting |
| Effective in small vessel injury | Essential in large vessel or deep injury |
🩺 Clinical Relevance
| Condition | Effect on Platelet Plug Formation |
|---|---|
| Thrombocytopenia | ↓ Platelet count → impaired plug formation |
| Aspirin use | Inhibits TXA₂ → ↓ platelet aggregation |
| von Willebrand disease | Defective vWF → ↓ adhesion |
| Glanzmann’s thrombasthenia | Defective GPIIb/IIIa → ↓ aggregation |
✅
Platelet plug formation is a fast, highly regulated process that prevents excessive blood loss after vascular injury. It relies on:
- Platelet adhesion via vWF
- Activation via ADP, TXA₂, and serotonin
- Aggregation via fibrinogen and GPIIb/IIIa receptors
This step is essential for initiating coagulation, especially in microvascular injuries, and any disruption can lead to bleeding disorders.
🧪 Coagulation Factors
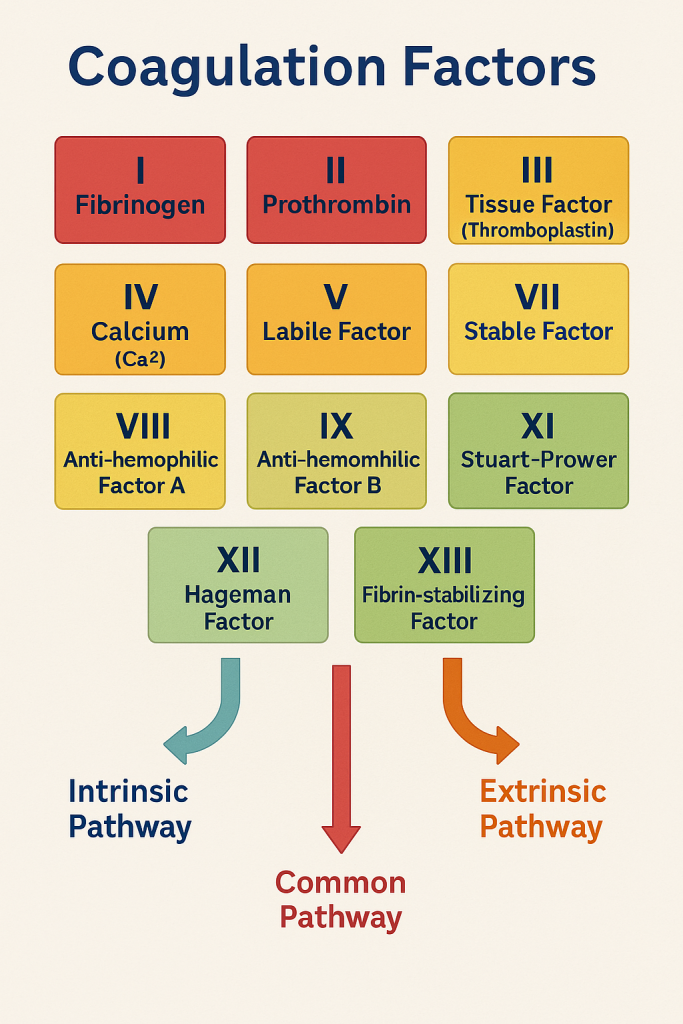
Coagulation factors (also known as clotting factors) are a group of proteins—mostly enzymes—that work in a coordinated cascade to form a stable blood clot in response to vascular injury. They are primarily produced by the liver, with some requiring vitamin K for synthesis.
These factors are involved in the coagulation cascade, which consists of intrinsic, extrinsic, and common pathways.
📜 List of Coagulation Factors
| Factor | Name | Function |
|---|---|---|
| I | Fibrinogen | Converted to fibrin by thrombin |
| II | Prothrombin | Converted to thrombin, a key enzyme in clotting |
| III | Tissue Factor (Thromboplastin) | Triggers extrinsic pathway |
| IV | Calcium (Ca²⁺) | Essential cofactor in many clotting reactions |
| V | Labile Factor | Cofactor for prothrombinase complex |
| VII | Stable Factor | Initiates extrinsic pathway with tissue factor |
| VIII | Anti-hemophilic Factor A | Cofactor in intrinsic pathway (deficient in Hemophilia A) |
| IX | Anti-hemophilic Factor B | Also called Christmas factor (deficient in Hemophilia B) |
| X | Stuart-Prower Factor | Point of convergence for intrinsic and extrinsic pathways |
| XI | Plasma Thromboplastin Antecedent | Involved in intrinsic pathway |
| XII | Hageman Factor | Activates Factor XI and fibrinolysis |
| XIII | Fibrin-stabilizing Factor | Cross-links fibrin to stabilize the clot |
🧬 Factors I, II, V, VII, VIII, IX, X, XI, XII, and XIII are proteins; Factor IV is calcium.
🔑 Vitamin K–Dependent Factors
- Factors II, VII, IX, X
- Require vitamin K for synthesis in the liver
- Blocked by warfarin (a vitamin K antagonist)
🔬 Pathways Involving Coagulation Factors
🔹 Intrinsic Pathway
Activated by damage inside the vessel
Involves: XII, XI, IX, VIII
🔹 Extrinsic Pathway
Activated by external trauma
Involves: III (Tissue Factor), VII
🔸 Common Pathway
Point of convergence
Involves: X, V, II (prothrombin), I (fibrinogen), XIII
🧪 Tests Related to Clotting Factors
| Test | Measures | Clinical Use |
|---|---|---|
| PT (Prothrombin Time) | Factors I, II, V, VII, X (Extrinsic + Common) | Monitors warfarin therapy |
| aPTT (Activated Partial Thromboplastin Time) | Factors I, II, V, VIII, IX, X, XI, XII | Monitors heparin therapy |
| TT (Thrombin Time) | Conversion of fibrinogen to fibrin | Screens for dysfibrinogenemia |
🧾 Quick Reference Mnemonic
“Foolish People Try Climbing Long Slopes After Christmas Some People Have Fallen.”
| Word | Factor |
|---|---|
| Foolish | Factor I (Fibrinogen) |
| People | Factor II (Prothrombin) |
| Try | Factor III (Tissue Factor) |
| Climbing | Factor IV (Calcium) |
| Long | Factor V (Labile Factor) |
| Slopes | Factor VII (Stable Factor) |
| After | Factor VIII (Anti-hemophilic A) |
| Christmas | Factor IX (Anti-hemophilic B) |
| Some | Factor X (Stuart-Prower) |
| People | Factor XI (PTA) |
| Have | Factor XII (Hageman) |
| Fallen | Factor XIII (Fibrin-stabilizing) |
✅
Coagulation factors are vital for initiating, amplifying, and stabilizing blood clot formation. Any deficiency or dysfunction in these factors can lead to bleeding disorders like hemophilia, vitamin K deficiency bleeding, or DIC.
🩸 Intrinsic and Extrinsic Pathways of Coagulation –
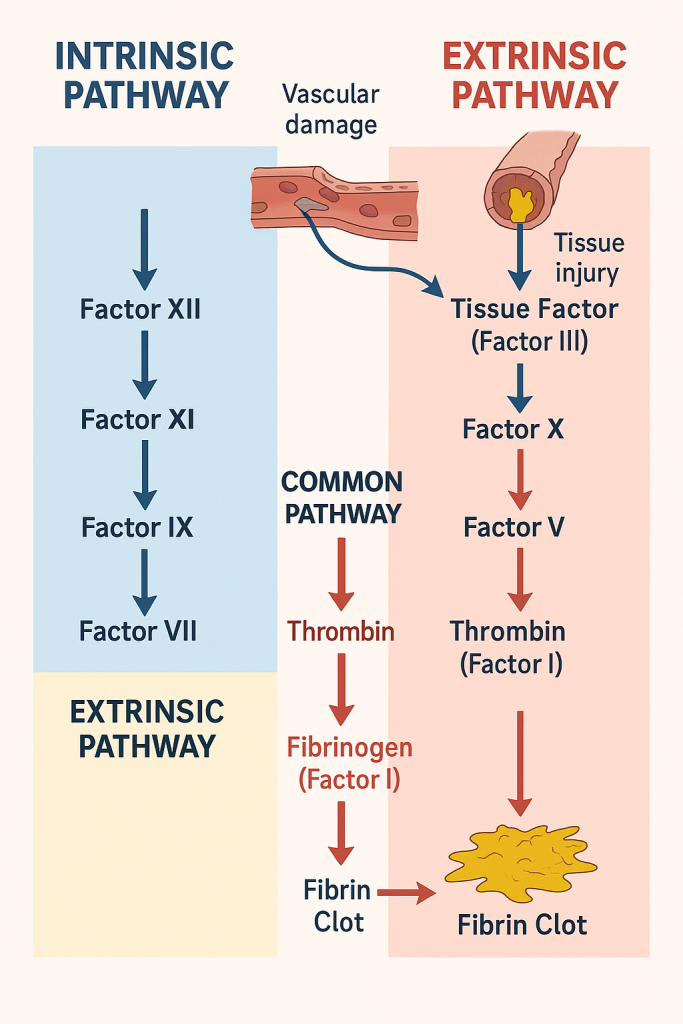
The coagulation cascade is the series of enzymatic reactions that leads to the formation of a fibrin clot. It is classically divided into:
- Intrinsic pathway (initiated by trauma inside the vascular system)
- Extrinsic pathway (initiated by external trauma to the vessel)
- Both converge into the common pathway, leading to fibrin clot formation
🔹 I. Intrinsic Pathway
✅ Definition:
Activated by endothelial injury or exposure of blood to subendothelial collagen, without the involvement of tissue factor.
🧬 Key Characteristics:
- All factors required are present within the blood (“intrinsic”)
- Slower activation (2–6 minutes)
- Tested by aPTT (Activated Partial Thromboplastin Time)
🧪 Steps:
- Factor XII (Hageman factor) is activated on contact with negatively charged surfaces (e.g., collagen).
- XIIa activates Factor XI.
- XIa activates Factor IX.
- IXa, with cofactor VIIIa, calcium, and phospholipids, activates Factor X (starting the common pathway).
🔍 Factors Involved: XII, XI, IX, VIII
🔸 II. Extrinsic Pathway
✅ Definition:
Triggered by external trauma that leads to the release of Tissue Factor (TF), also known as Factor III, from damaged tissues.
🧬 Key Characteristics:
- Involves elements outside of the blood (“extrinsic”)
- Rapid response (15–20 seconds)
- Tested by PT (Prothrombin Time) and INR in patients on warfarin
🧪 Steps:
- Tissue Factor (Factor III) is released from injured tissues.
- It binds with Factor VII to form a TF-VIIa complex.
- This complex, in the presence of calcium, directly activates Factor X (common pathway).
🔍 Factors Involved: III (TF), VII
🔀 III. Common Pathway
Both intrinsic and extrinsic pathways converge here:
- Factor X is activated (Xa).
- Xa combines with Factor V, calcium, and phospholipids to form prothrombinase.
- Prothrombinase converts prothrombin (Factor II) → thrombin.
- Thrombin converts fibrinogen (Factor I) → fibrin.
- Factor XIII stabilizes the fibrin clot.
🔍 Factors Involved: X, V, II (prothrombin), I (fibrinogen), XIII
🧾 Comparison Table: Intrinsic vs Extrinsic Pathway
| Feature | Intrinsic Pathway | Extrinsic Pathway |
|---|---|---|
| Trigger | Endothelial damage, exposed collagen | Tissue injury releasing TF |
| Initiating Factor | Factor XII | Factor III (Tissue Factor) + Factor VII |
| Speed | Slower (minutes) | Faster (seconds) |
| Lab Test | aPTT (Activated Partial Thromboplastin Time) | PT (Prothrombin Time), INR |
| Clotting Factors | XII, XI, IX, VIII | III, VII |
| Used to Monitor | Heparin therapy | Warfarin therapy |
🩺 Clinical Significance
| Condition | Pathway Affected | Lab Findings |
|---|---|---|
| Hemophilia A (Factor VIII deficiency) | Intrinsic | ↑ aPTT |
| Hemophilia B (Factor IX deficiency) | Intrinsic | ↑ aPTT |
| Vitamin K deficiency | Both (affects II, VII, IX, X) | ↑ PT and aPTT |
| Liver disease | All pathways | ↑ PT and aPTT |
| Disseminated Intravascular Coagulation (DIC) | All pathways | ↑ PT, ↑ aPTT, ↓ platelets, ↓ fibrinogen |
✅
Both the intrinsic and extrinsic pathways are critical to initiating the coagulation cascade. While they differ in their triggers and speed, they converge on the common pathway to produce thrombin, which is essential for fibrin clot formation.
Understanding these pathways is vital for interpreting coagulation tests, diagnosing bleeding disorders, and managing patients on anticoagulants.
🩸 Blood Groups and Types – Academic Overview
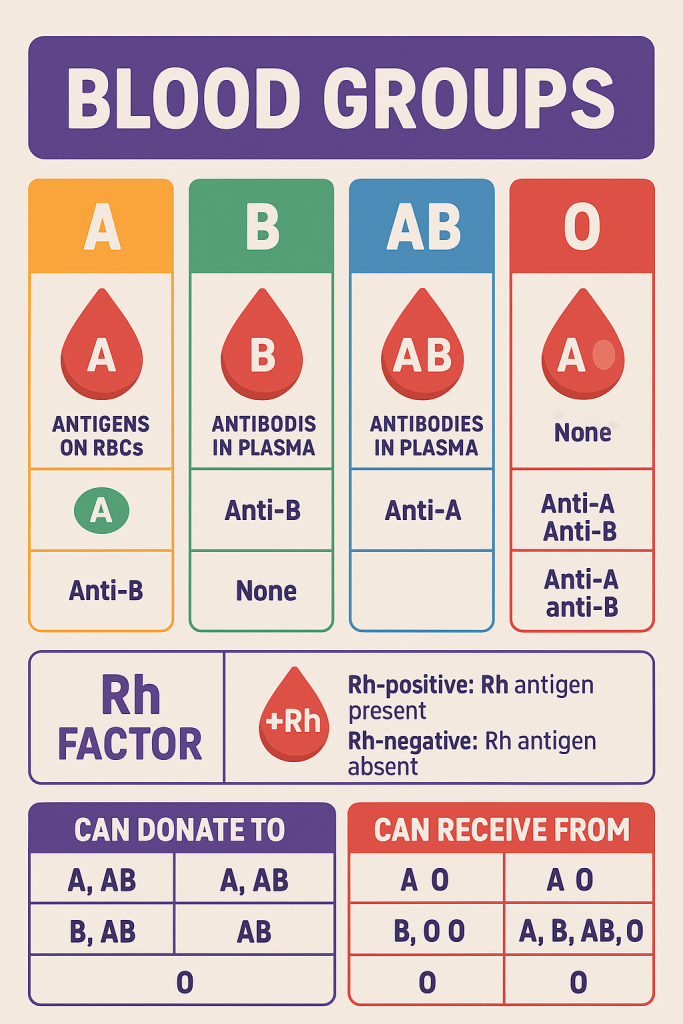
Blood grouping is the classification of blood based on the presence or absence of specific antigens on the surface of red blood cells (RBCs) and the corresponding antibodies in the plasma. It is crucial for safe blood transfusions, organ transplantation, and in prenatal care.
The two most important blood group systems are:
- ABO system
- Rh (Rhesus) system
🧬 I. ABO Blood Group System
The ABO system is based on the presence or absence of two antigens—A and B—on the surface of RBCs, and the presence of anti-A and/or anti-B antibodies in the plasma.
🔍 Blood Types in the ABO System
| Blood Group | RBC Antigens | Plasma Antibodies | Can Donate To | Can Receive From |
|---|---|---|---|---|
| A | A antigen | Anti-B | A, AB | A, O |
| B | B antigen | Anti-A | B, AB | B, O |
| AB | A and B antigens | None | AB | A, B, AB, O (Universal recipient) |
| O | None | Anti-A and Anti-B | A, B, AB, O (Universal donor) | O |
🧠 II. Rh (Rhesus) Blood Group System
- Based on the presence (Rh-positive) or absence (Rh-negative) of the Rh (D) antigen on RBCs.
- Rh+ individuals have the D antigen, and Rh– individuals do not.
- Anti-D antibodies are not naturally present but can develop after:
- Exposure through transfusion
- Rh incompatibility during pregnancy (mother Rh–, fetus Rh+)
🩺 Clinical Relevance – Rh Incompatibility in Pregnancy
- Erythroblastosis fetalis or Hemolytic Disease of the Newborn (HDN) occurs when maternal anti-D antibodies cross the placenta and destroy fetal RBCs.
- Prevented using Rh immunoglobulin (RhIg or Rho(D) immune globulin) during and after pregnancy.
🧾 III. Summary of ABO & Rh Blood Types
| Blood Type | Antigens on RBCs | Antibodies in Plasma | % Population (approx.) |
|---|---|---|---|
| A+ | A, Rh(D) | Anti-B | ~34% |
| A– | A | Anti-B, possibly Anti-D | ~6% |
| B+ | B, Rh(D) | Anti-A | ~9% |
| B– | B | Anti-A, possibly Anti-D | ~2% |
| AB+ | A, B, Rh(D) | None | ~4% |
| AB– | A, B | Possibly Anti-D | ~1% |
| O+ | None, Rh(D) | Anti-A, Anti-B | ~37% |
| O– | None | Anti-A, Anti-B, possibly Anti-D | ~7% |
🔖 O– is the universal donor for RBCs, AB+ is the universal recipient.
🔬 IV. Blood Transfusion Rules
- Blood transfusions must match ABO and Rh compatibility.
- Mismatched transfusions can cause hemolytic transfusion reactions, which are life-threatening.
🛑 Example of Danger:
- Giving B blood to a person with type A:
- Their Anti-B antibodies attack the donor RBCs → hemolysis, shock, possible death
🧪 V. Cross-Matching and Testing
Before a transfusion:
- Blood typing: Determine ABO and Rh status
- Cross-matching: Test recipient serum with donor RBCs for compatibility
- Indirect Coombs test: Detects antibodies in recipient’s serum
- Direct Coombs test: Detects antibodies on RBCs
🩺 VI. Clinical Importance of Blood Groups
| Application | Purpose |
|---|---|
| Blood transfusion | Avoids incompatible transfusions |
| Organ transplantation | Minimizes rejection risk |
| Pregnancy care | Manages Rh incompatibility |
| Forensic science and paternity testing | Identifies relationships or individuals |
| Blood bank storage and management | Ensures inventory of all blood types |
✅
Blood groups are determined by genetically inherited antigens on RBCs. Understanding the ABO and Rh systems is essential for safe transfusion, prenatal care, and medical emergencies. Mismatched blood transfusions can be fatal, highlighting the importance of proper blood typing and cross-matching.
🩸 Procedure to Identify ABO and Rh (BGRH) Blood Group
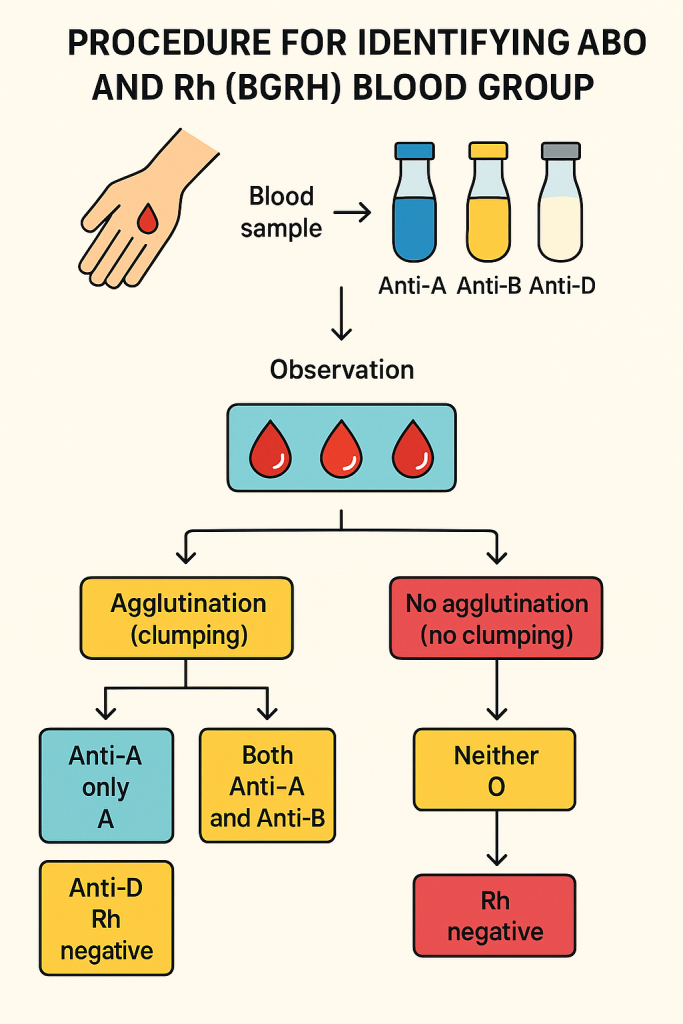
The process is known as blood grouping or forward typing and involves testing a person’s RBC antigens with known antisera (antibodies). The most common and rapid method is the slide method or tube method.
🧪 I. Materials Required
- Clean glass slides or test tubes
- Antisera:
- Anti-A (blue)
- Anti-B (yellow)
- Anti-D (clear) (for Rh typing)
- Sterile lancet or sample of anticoagulated blood
- Mixing sticks or applicators
- Normal saline
- Microscope (if needed for confirmation)
📋 II. Procedure (Slide Method – Quick Field Test)
Step 1: Labeling
- Label the slide into three sections: Anti-A, Anti-B, Anti-D
Step 2: Add Antisera
- Place 1 drop of:
- Anti-A serum in section A
- Anti-B serum in section B
- Anti-D serum in section D
Step 3: Add Blood Sample
- Add one drop of blood to each serum drop using a separate applicator or pipette.
- Mix each well with a fresh stick or applicator.
Step 4: Observation
- Gently rock the slide for 1–2 minutes and observe agglutination (clumping).
🔍 III. Interpretation
| Agglutination with | Blood Group |
|---|---|
| Anti-A only | A |
| Anti-B only | B |
| Both Anti-A and Anti-B | AB |
| Neither | O |
| Agglutination with Anti-D | Rh Type |
|---|---|
| Yes | Rh positive |
| No | Rh negative |
🔖 Example: Agglutination with Anti-B and Anti-D → B positive
🧪 Tube Method (More Sensitive – Lab Setting)
- Prepare a 5% suspension of patient’s RBCs in saline.
- Label 3 test tubes: Anti-A, Anti-B, Anti-D.
- Add 2 drops of each antiserum into respective tubes.
- Add 1 drop of RBC suspension into each.
- Mix, centrifuge for 15–30 seconds.
- Observe for agglutination by gently resuspending the cells.
⚠️ Precautions
- Use clean, dry equipment to avoid cross-reactions.
- Do not delay reading results—agglutination may disappear.
- Ensure antisera are not expired.
- In case of weak or unclear reactions, perform reverse grouping (test plasma with known RBCs).
🧬 IV. Reverse Grouping (Plasma Testing – Confirmatory)
- Mix patient’s plasma with known A and B RBCs
- Agglutination occurs if corresponding antibodies are present
- Helps confirm forward typing results
✅
Blood group and Rh typing is a simple, rapid, yet critical procedure in:
- Blood transfusion safety
- Organ transplantation
- Antenatal care
- Emergency management
It ensures donor-recipient compatibility and prevents life-threatening transfusion reactions.
🧠 Reticuloendothelial System (RES) –
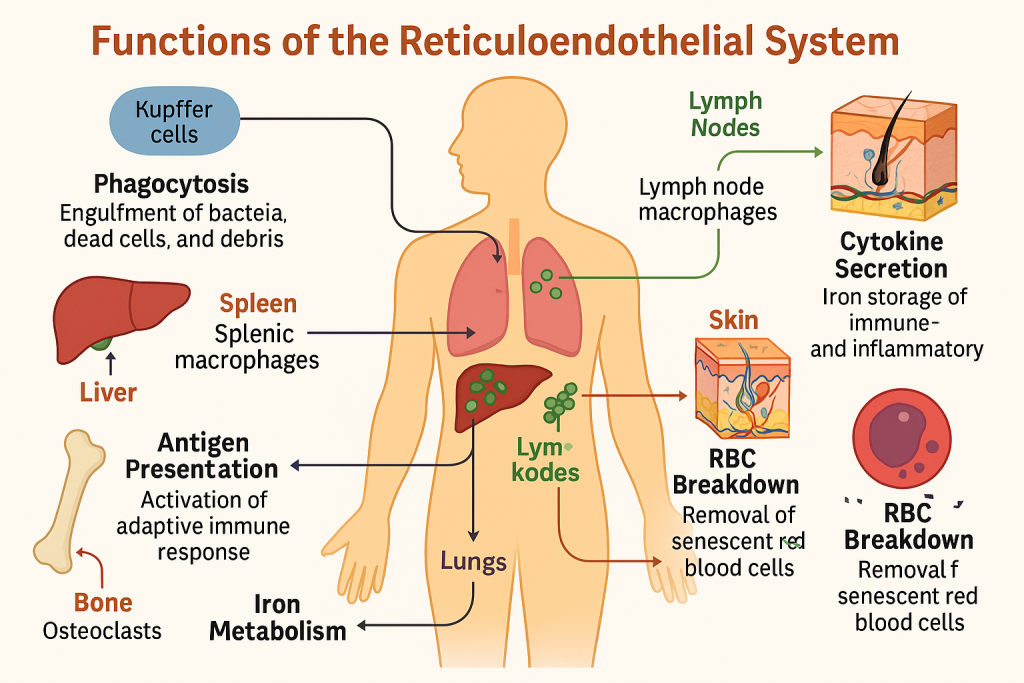
The reticuloendothelial system (RES) is a network of phagocytic cells located in various tissues, responsible for recognizing, engulfing, and destroying foreign substances, dead cells, and pathogens. It is now more accurately referred to as the mononuclear phagocyte system (MPS) due to its cellular components.
🔬 I. Components of the RES
The system is made up of monocytes in the blood and macrophages in tissues. These include:
| Cell Type | Location | Special Name |
|---|---|---|
| Monocytes | Blood circulation | — |
| Macrophages | General tissue | Tissue macrophages |
| Kupffer cells | Liver | Specialized hepatic macrophages |
| Alveolar macrophages | Lungs | Dust cells |
| Microglia | Central nervous system | Brain macrophages |
| Langerhans cells | Skin | Dendritic antigen-presenting cells |
| Splenic macrophages | Spleen (red pulp) | — |
| Lymph node macrophages | Lymphatic tissue | — |
| Osteoclasts | Bone | Bone-resorbing macrophages |
🩺 II. Functions of the Reticuloendothelial System
1. Phagocytosis
- Primary function of RES.
- Engulfs and digests:
- Bacteria
- Viruses
- Dead or damaged cells
- Cellular debris
- Opsonization enhances phagocytosis via antibodies or complement proteins.
2. Antigen Processing and Presentation
- Macrophages process ingested foreign particles.
- Present antigens via MHC class II molecules to T-helper cells.
- Initiates adaptive immune response.
3. Clearance of Senescent Cells
- RES removes aged or damaged RBCs and platelets, especially in the spleen and liver.
- Iron and amino acids from hemoglobin are recycled.
4. Immune Surveillance
- Constantly monitors tissues for pathogens, tumor cells, and antigens.
- Helps in early detection and immune activation.
5. Cytokine Secretion
- Produces pro-inflammatory and anti-inflammatory cytokines:
- IL-1, IL-6, TNF-α → stimulate inflammation and fever
- Stimulates leukocyte recruitment and immune modulation
6. Iron Storage and Recycling
- Macrophages store iron as ferritin and hemosiderin.
- Release iron when needed for new hemoglobin synthesis.
7. Tissue Repair and Wound Healing
- Macrophages secrete:
- Growth factors (e.g., VEGF, PDGF)
- Stimulate angiogenesis and fibroblast proliferation
- Clean up debris in wound sites to promote healing
8. Removal of Immune Complexes
- RES in the liver and spleen removes antigen–antibody complexes.
- Prevents tissue damage from immune complex deposition.
🧬 III. Summary Table of RES Functions
| Function | Description |
|---|---|
| Phagocytosis | Engulfment and digestion of pathogens and debris |
| Antigen presentation | Activation of T-cells for adaptive immunity |
| RBC breakdown | Splenic/liver macrophages recycle iron and remove aged RBCs |
| Cytokine secretion | Modulates inflammation and immune response |
| Iron storage | Stores iron for hemoglobin production |
| Immune surveillance | Monitors tissues for antigens and abnormal cells |
| Removal of immune complexes | Clears harmful circulating antigen–antibody complexes |
| Tissue repair | Releases factors that assist in wound healing and tissue regeneration |
🩺 IV. Clinical Relevance
| Condition | Impact on RES Function |
|---|---|
| Sepsis | Hyperactivation of macrophages → systemic inflammation |
| HIV/AIDS | Impaired macrophage–T cell interaction |
| Splenectomy | Loss of key RES organ → risk of infection |
| Gaucher’s disease | Defect in macrophage lysosomal enzyme → accumulation |
| Iron overload (hemochromatosis) | Overwhelms macrophage storage capacity |
| Autoimmune diseases | Defective clearance of immune complexes |
✅
The reticuloendothelial system is essential for:
- Innate immunity
- Clearance of senescent cells
- Antigen presentation
- Inflammatory response
- Tissue healing and iron metabolism
Its proper function is critical for maintaining immune homeostasis, and its dysfunction can lead to infection, autoimmunity, or impaired healing.
🛡️ Functions of Immunity – Academic Overview
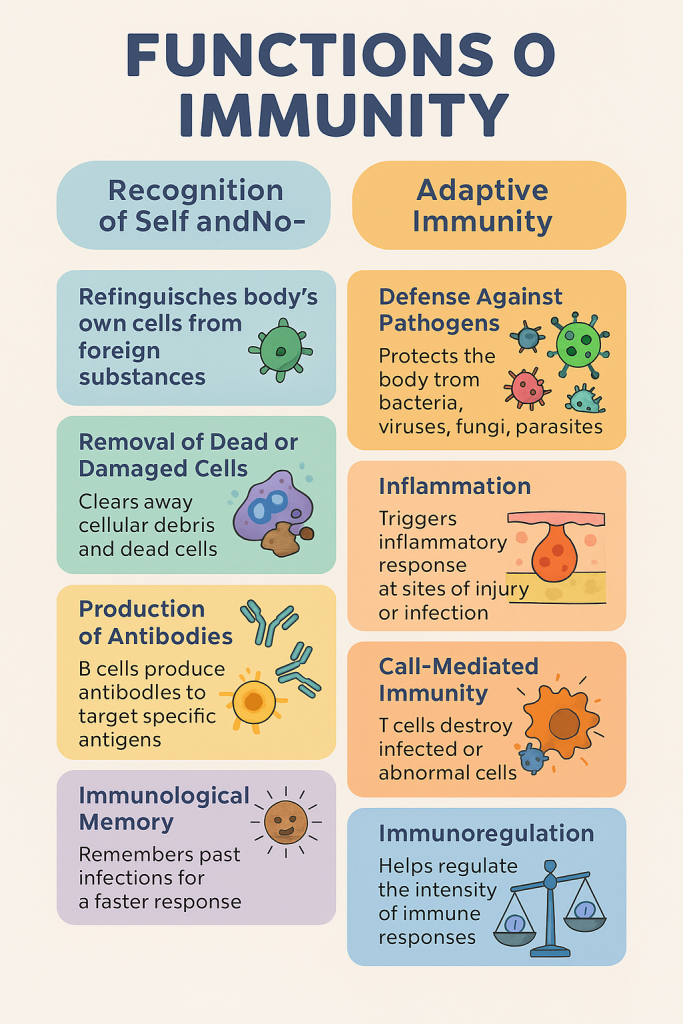
Immunity refers to the body’s ability to recognize, resist, and eliminate pathogens, harmful substances, and abnormal cells. It is a vital defense mechanism that maintains homeostasis and protects the body from infections, cancer, and foreign substances.
Immunity is carried out through a complex network of organs, cells, and molecules organized into innate and adaptive systems.
🔬 I. Types of Immunity
| Type | Description | Onset | Specificity | Memory |
|---|---|---|---|---|
| Innate | Present at birth, non-specific | Immediate | Non-specific | No |
| Adaptive | Acquired after exposure to specific antigens | Delayed | Highly specific | Yes |
🧠 II. Main Functions of Immunity
1. Recognition of Self and Non-Self
- Immune cells distinguish between own body cells (self) and foreign invaders (non-self).
- Achieved through recognition of antigens using receptors on immune cells (e.g., T-cell receptors, antibodies).
2. Defense Against Pathogens
- Protects against bacteria, viruses, fungi, and parasites via:
- Physical barriers (skin, mucosa)
- Phagocytes (neutrophils, macrophages)
- Antibodies and complement
- Cytotoxic T-cells and NK cells
3. Removal of Dead and Damaged Cells
- Phagocytic cells clean up cellular debris, dead cells, and damaged tissue.
- Important in tissue repair and regeneration.
4. Inflammation
- Immune response triggers inflammation at injury or infection sites:
- Redness, swelling, heat, pain, loss of function
- Aims to contain infection, recruit immune cells, and begin healing.
5. Production of Antibodies (Humoral Immunity)
- B-lymphocytes produce antibodies against specific antigens.
- Antibodies:
- Neutralize toxins
- Promote phagocytosis (opsonization)
- Activate the complement system
6. Cell-Mediated Immunity
- T-lymphocytes directly destroy infected, cancerous, or foreign cells.
- Important in:
- Viral infections
- Organ transplant rejection
- Tumor surveillance
7. Immunological Memory
- Memory B and T cells are formed after exposure to an antigen.
- They enable a faster and stronger response upon re-exposure.
- Basis of vaccination.
8. Immunoregulation
- Regulates intensity and duration of immune responses.
- Regulatory T cells (Tregs) prevent autoimmunity by suppressing overactive immune responses.
9. Surveillance Against Malignancy
- Immune cells (especially cytotoxic T cells and NK cells) identify and destroy abnormal or cancerous cells before tumors form.
10. Activation of Complement System
- A cascade of plasma proteins that:
- Lyse pathogens
- Promote inflammation
- Enhance phagocytosis
🩺 III. Clinical Relevance of Immune Functions
| Condition | Related Immune Dysfunction |
|---|---|
| Immunodeficiency (e.g., HIV) | ↓ Defense against infections |
| Autoimmune diseases | Failure to recognize self |
| Allergies | Exaggerated response to harmless antigens |
| Cancer | Failure in immune surveillance |
| Organ rejection | Excessive cell-mediated immunity |
| Vaccination | Based on immunological memory |
🧾 Summary Table of Immune Functions
| Function | Key Cells Involved | Purpose |
|---|---|---|
| Self/non-self recognition | T cells, APCs | Maintain tolerance |
| Pathogen elimination | Neutrophils, macrophages, T cells | Prevent infection |
| Antibody production | B cells, plasma cells | Neutralize and eliminate antigens |
| Inflammation | Mast cells, cytokines, complement | Recruit and activate immune cells |
| Memory generation | Memory B & T cells | Faster response to repeated infections |
| Tumor surveillance | NK cells, cytotoxic T cells | Destroy mutated cells |
| Regulation of response | Regulatory T cells | Prevent autoimmunity and overreaction |
✅
The immune system performs critical protective, regulatory, and restorative functions, defending the body against infections and abnormal cells, while maintaining tolerance and homeostasis. Dysfunction of these functions leads to infection, cancer, autoimmune diseases, or hypersensitivity reactions.
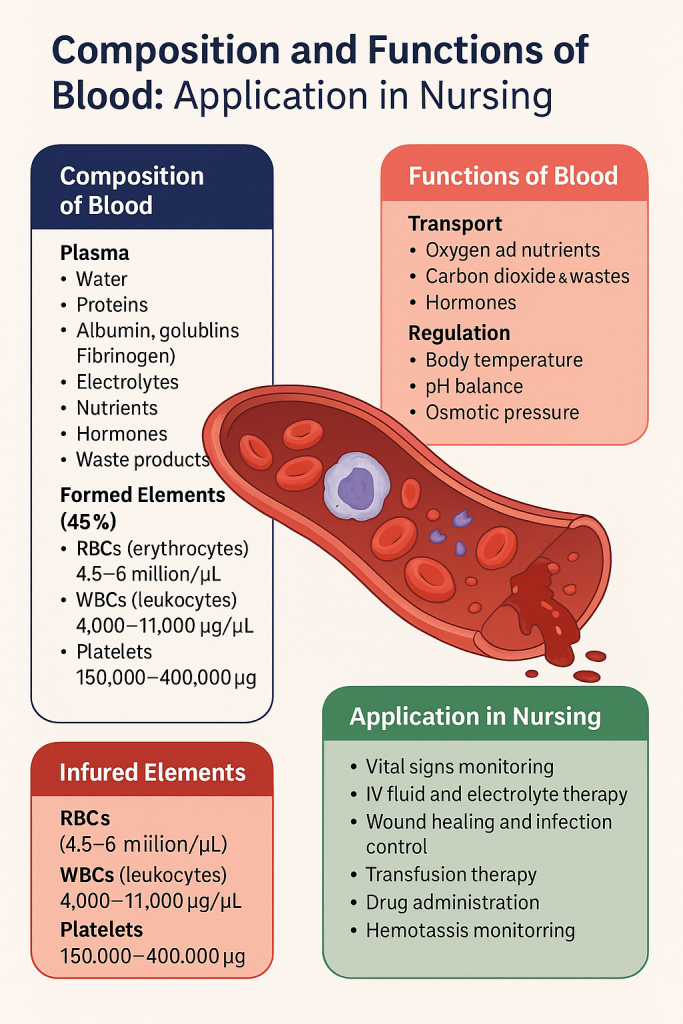
🩸 Composition and Functions of Blood – With Nursing Applications
Blood is a specialized connective tissue vital to human life. It performs key roles in transport, regulation, and protection. Understanding its composition and function is essential for nurses to assess, monitor, and manage various clinical conditions.
🧪 I. Composition of Blood
Blood is made up of two main components:
1. Plasma (55%) – The liquid portion
| Component | Function |
|---|---|
| Water (~92%) | Solvent, temperature regulation, transport medium |
| Plasma proteins | Albumin (osmotic pressure), Globulins (immunity), Fibrinogen (clotting) |
| Electrolytes | Na⁺, K⁺, Ca²⁺, HCO₃⁻ – essential for nerve and muscle function |
| Nutrients | Glucose, amino acids, lipids – cellular metabolism |
| Hormones & Enzymes | Regulation of body functions |
| Waste products | Urea, creatinine – excreted by kidneys |
2. Formed Elements (45%) – The cellular portion
| Cell Type | Normal Range | Function |
|---|---|---|
| RBCs (Erythrocytes) | 4.5–6 million/μL | Transport O₂ and CO₂ via hemoglobin |
| WBCs (Leukocytes) | 4,000–11,000/μL | Defense against infection |
| Platelets (Thrombocytes) | 150,000–400,000/μL | Blood clotting and vessel repair |
🧠 II. Functions of Blood
🔹 1. Transport
- Oxygen and nutrients to tissues
- Carbon dioxide and waste to excretory organs
- Hormones to target organs
🔹 2. Regulation
- Body temperature
- pH balance through buffer systems
- Osmotic pressure and fluid balance
🔹 3. Protection
- WBCs defend against infection
- Platelets and clotting factors prevent blood loss
- Antibodies and complement enhance immunity
🩺 III. Application in Nursing Practice
| Nursing Area | Relevance of Blood Composition and Function |
|---|---|
| Vital signs monitoring | Detect changes in perfusion and oxygenation (e.g., anemia, hypoxia) |
| IV fluid and electrolyte therapy | Requires knowledge of plasma electrolytes and osmotic balance |
| Wound healing and infection control | WBC count and immune response guide antibiotic therapy |
| Transfusion therapy | Requires understanding of blood components, compatibility, reactions |
| Drug administration | Plasma protein levels affect drug binding and bioavailability |
| Hemostasis monitoring | Platelet counts and clotting factors guide bleeding control |
| Nutritional assessment | RBCs reflect iron, B12, and folate status |
| Pre and post-operative care | Monitoring for anemia, infection risk, and clotting abnormalities |
🧾 Examples in Clinical Practice
- Anemia: Monitor hemoglobin, assess fatigue and pallor, ensure iron therapy.
- Dehydration: Increased hematocrit due to low plasma volume.
- Infection: Elevated WBCs → initiate antibiotic therapy.
- Bleeding disorder: Low platelets or clotting factors → risk of hemorrhage.
- Hypoproteinemia: Low albumin → edema, requires dietary or IV correction.
✅
A comprehensive understanding of the composition and functions of blood is essential for nurses to:
- Assess patient conditions
- Plan appropriate interventions
- Interpret lab reports
- Monitor fluid-electrolyte status
- Manage transfusions and medications
🩺 Blood – Applications in Nursing
Blood plays a central role in physiological function, and understanding its composition, functions, and disorders is fundamental to nursing care. Nurses encounter and manage blood-related scenarios across a wide range of settings—from routine monitoring to emergency interventions.
🧠 I. Clinical Assessment and Monitoring
1. Vital Signs Interpretation
- Blood pressure, pulse, temperature, and oxygen saturation reflect blood flow, volume, and oxygenation.
- Recognizing abnormalities (e.g., hypotension, tachycardia) helps detect hypovolemia, anemia, or shock.
2. Observation of Skin and Mucous Membranes
- Pallor → anemia
- Cyanosis → hypoxemia
- Petechiae or bruising → platelet dysfunction or clotting disorders
3. Monitoring Capillary Refill Time
- Assesses peripheral perfusion in critical care and emergency settings.
🧪 II. Interpretation of Laboratory Tests
Nurses frequently monitor and interpret blood investigations:
| Test | Nursing Application |
|---|---|
| Hemoglobin (Hb) | Detects anemia or bleeding |
| WBC Count | Indicates infection or immune suppression |
| Platelet Count | Assesses bleeding risk, thrombocytopenia |
| Coagulation Profile (PT, aPTT, INR) | Monitors clotting status, esp. during anticoagulant therapy |
| Blood Grouping & Cross-Matching | Essential before transfusions |
💉 III. Blood Transfusion
Nurses play a vital role in safe transfusion practices:
1. Preparation and Verification
- Ensure correct blood group compatibility
- Verify patient identity, blood bag, and physician order
2. Monitoring During Transfusion
- Check vital signs pre-, intra-, and post-transfusion
- Watch for transfusion reactions:
- Fever, chills, rash
- Hypotension, back pain, dyspnea (signs of hemolytic reaction)
3. Documentation and Reporting
- Record time, type, volume, and any reactions or interventions
🚨 IV. Emergency and Critical Care
Nurses manage blood-related emergencies like:
- Hemorrhage → rapid recognition, pressure application, fluid/blood administration
- Shock → initiate IV access, administer oxygen, monitor perfusion
- Sepsis → monitor CBC, blood cultures, support organ perfusion
🩹 V. Perioperative and Postoperative Care
- Monitor for bleeding, coagulopathy, or fluid loss
- Manage postoperative anemia or blood replacement therapy
- Administer and monitor anticoagulants post-surgery
👶 VI. Maternal and Neonatal Nursing
- Rh incompatibility management using anti-D injection
- Monitor for anemia in pregnancy
- Ensure cross-matching during delivery if hemorrhage occurs
- Observe neonates for jaundice or blood-related genetic disorders
🧬 VII. Health Education and Patient Counseling
- Educate patients on:
- Blood donation
- Iron-rich diets in anemia
- Medication adherence in anticoagulant therapy
- Infection precautions in immunocompromised states
🧾 VIII. Summary Table: Nursing Applications of Blood
| Domain | Application |
|---|---|
| Assessment | Vital signs, pallor, perfusion, bleeding signs |
| Diagnostics | CBC, ESR, coagulation tests, blood cultures |
| Interventions | Transfusion, oxygen therapy, IV fluids |
| Monitoring | Post-surgical bleeding, transfusion reactions |
| Emergency Care | Hemorrhage, sepsis, shock |
| Health Promotion | Blood donation, nutrition, vaccination |
✅
Blood is not only the medium of life but also a clinical indicator, therapeutic target, and intervention point in nursing. A nurse’s ability to assess, interpret, and respond to blood-related changes directly affects patient outcomes in both acute and chronic care settings.