BSC SEM 1 UNIT 4 APPLIED BIOCHEMISTRY
UNIT 4 Clinical Enzymology
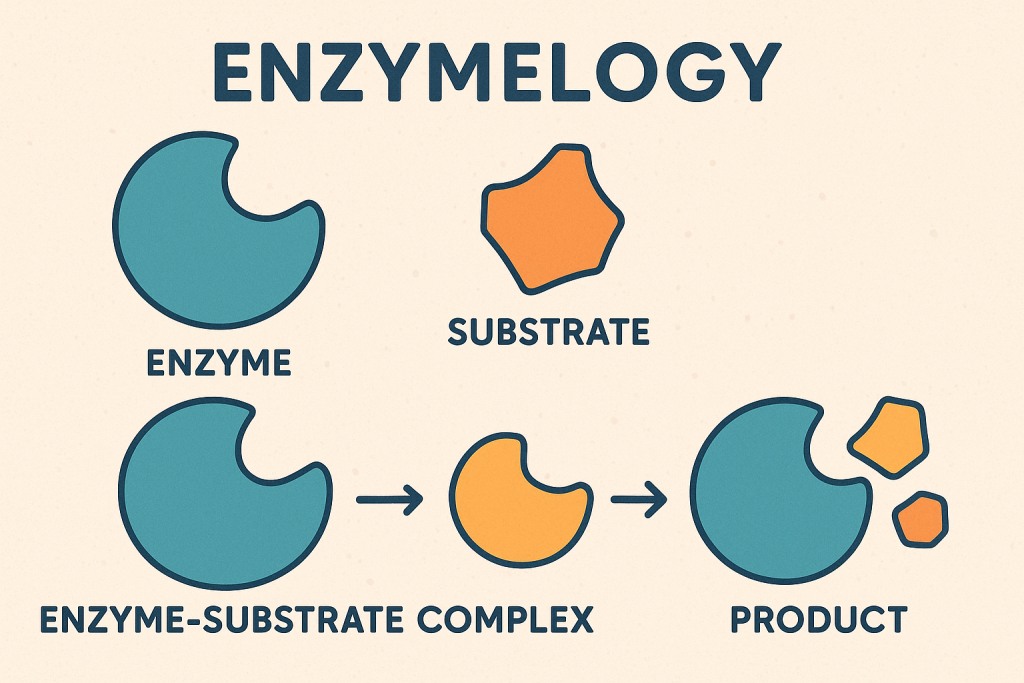
Clinical Enzymology:
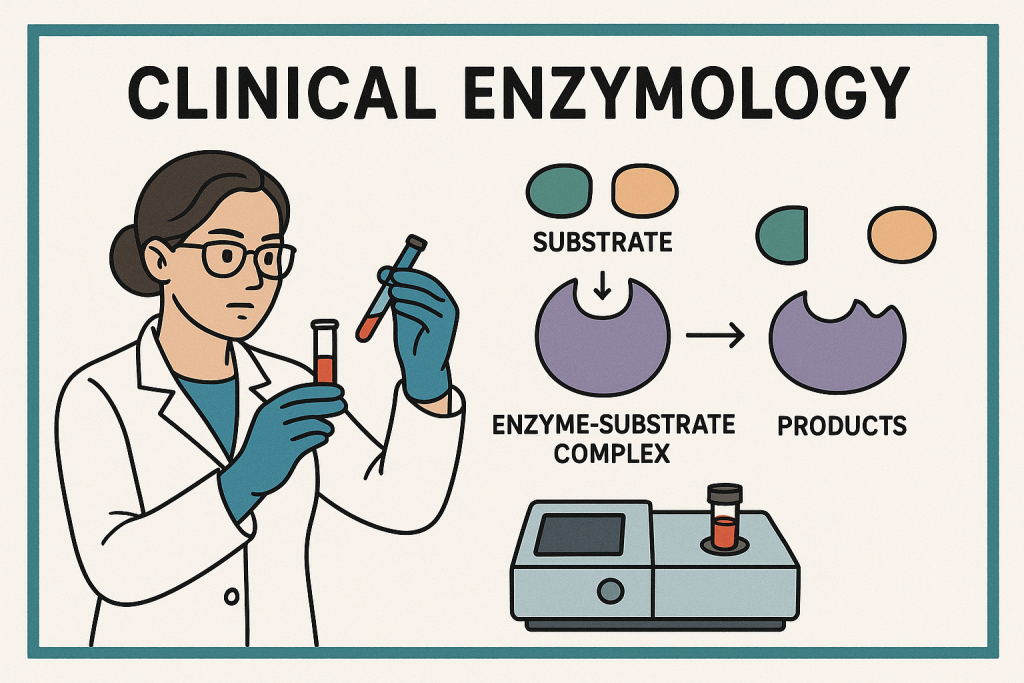
Introduction to Clinical Enzymology
Clinical enzymology is the branch of biochemistry that deals with the role of enzymes in health and disease. It involves the measurement of enzyme activity in body fluids (such as blood, urine, and cerebrospinal fluid) to aid in the diagnosis, prognosis, and monitoring of various medical conditions.
Enzymes are biological catalysts that speed up biochemical reactions in the body. They are highly specific and play a crucial role in metabolism, digestion, and cellular functions. In clinical diagnostics, the measurement of enzyme activity helps detect tissue damage, organ dysfunction, and metabolic disorders.
1. Characteristics of Enzymes
- Biological Catalysts: Speed up chemical reactions without being consumed.
- Specificity: Act on specific substrates to produce a particular reaction.
- Sensitivity to pH and Temperature: Enzymes work best at an optimal pH and temperature.
- Regulation: Their activity can be increased or decreased by activators or inhibitors.
- Isoenzymes: Different molecular forms of the same enzyme found in different tissues.
2. Classification of Clinically Important Enzymes
Based on their diagnostic importance, clinical enzymes can be categorized into:
A. Diagnostic Enzymes (Marker Enzymes)
These enzymes indicate tissue or organ damage when found in abnormal levels in body fluids.
| Enzyme | Organ/System Associated | Clinical Significance |
|---|---|---|
| Alanine Aminotransferase (ALT/SGPT) | Liver | Hepatitis, liver damage |
| Aspartate Aminotransferase (AST/SGOT) | Liver, Heart, Muscle | Myocardial infarction, liver disease |
| Alkaline Phosphatase (ALP) | Liver, Bone | Bone disorders, liver disease |
| Acid Phosphatase (ACP) | Prostate | Prostate cancer marker |
| Lactate Dehydrogenase (LDH) | Heart, Liver, RBCs | Myocardial infarction, hemolysis |
| Creatine Kinase (CK/CPK) | Heart, Muscle, Brain | Myocardial infarction, muscle disorders |
| Gamma-Glutamyl Transferase (GGT) | Liver | Alcoholic liver disease |
| Amylase & Lipase | Pancreas | Pancreatitis |
| Cholinesterase (CHE) | Liver | Liver dysfunction, organophosphate poisoning |
B. Therapeutic Enzymes (Used in Treatment)
Certain enzymes are used as therapeutic agents to treat specific medical conditions.
| Enzyme | Medical Use |
|---|---|
| Streptokinase/Urokinase | Dissolves blood clots (Thrombolytic therapy) |
| L-Asparaginase | Treats leukemia (cancer therapy) |
| Pancreatic Enzymes (Lipase, Amylase, Protease) | Used in pancreatic insufficiency |
| Papain & Bromelain | Used in wound healing, digestion |
C. Genetic Enzyme Deficiencies
Some diseases result from genetic mutations affecting enzyme function.
| Enzyme Deficiency | Disease |
|---|---|
| Glucose-6-Phosphate Dehydrogenase (G6PD) | Hemolytic anemia |
| Phenylalanine Hydroxylase | Phenylketonuria (PKU) |
| Hexosaminidase A | Tay-Sachs disease |
| Galactose-1-Phosphate Uridyltransferase | Galactosemia |
3. Clinical Applications of Enzymes in Diagnosis
A. Liver Function Tests
- ALT (Alanine Aminotransferase): Specific for liver damage.
- AST (Aspartate Aminotransferase): Also found in heart and muscle, so less specific.
- ALP (Alkaline Phosphatase): Raised in liver disease and bone disorders.
- GGT (Gamma-Glutamyl Transferase): Increased in alcoholic liver disease.
B. Cardiac Enzymes for Myocardial Infarction (Heart Attack)
- Creatine Kinase-MB (CK-MB): Rises 4-6 hours after a heart attack.
- Lactate Dehydrogenase (LDH): Late marker, peaks in 2-3 days.
- Troponins (T & I): Most specific and sensitive markers for cardiac damage.
C. Pancreatic Enzymes for Pancreatitis
- Amylase: Increased in acute pancreatitis.
- Lipase: More specific for pancreatic damage than amylase.
D. Muscle Disorders (Muscular Dystrophy, Rhabdomyolysis)
- Creatine Kinase (CK-MM): Increased in muscle diseases.
- LDH: Also elevated in muscle injury.
E. Prostate Cancer Marker
- Acid Phosphatase (ACP): Elevated in prostate cancer.
F. Bone Disease Markers
- Alkaline Phosphatase (ALP): Increased in rickets, osteomalacia, and Paget’s disease.
4. Enzyme Kinetics in Clinical Diagnostics
- Michaelis-Menten Equation: Describes enzyme kinetics.
- Zero-Order Kinetics: Used in enzyme assays when the substrate is in excess.
- First-Order Kinetics: Used in drug metabolism.
5. Methods of Measuring Enzyme Activity in Clinical Laboratories
- Spectrophotometry: Measures the absorbance of light.
- Electrophoresis: Used for isoenzyme separation.
- Immunoassays: ELISA for enzyme detection.
- Chromatography: High-performance liquid chromatography (HPLC) for enzyme measurement.
6. Factors Affecting Enzyme Levels in the Body
- Physiological Factors: Age, gender, exercise, diet.
- Pathological Factors: Diseases, infections, genetic disorders.
- External Factors: Medications, toxins, temperature.
7. Recent Advances in Clinical Enzymology
- Enzyme Biomarkers in Cancer: Enzymes like MMPs (Matrix Metalloproteinases) in cancer detection.
- Enzyme-Based Biosensors: Used for glucose monitoring in diabetes.
- Gene Therapy: Correction of enzyme deficiencies at the genetic level.
- CRISPR Technology: Gene editing to restore enzyme function.
Isoenzymes (Isozymes) –
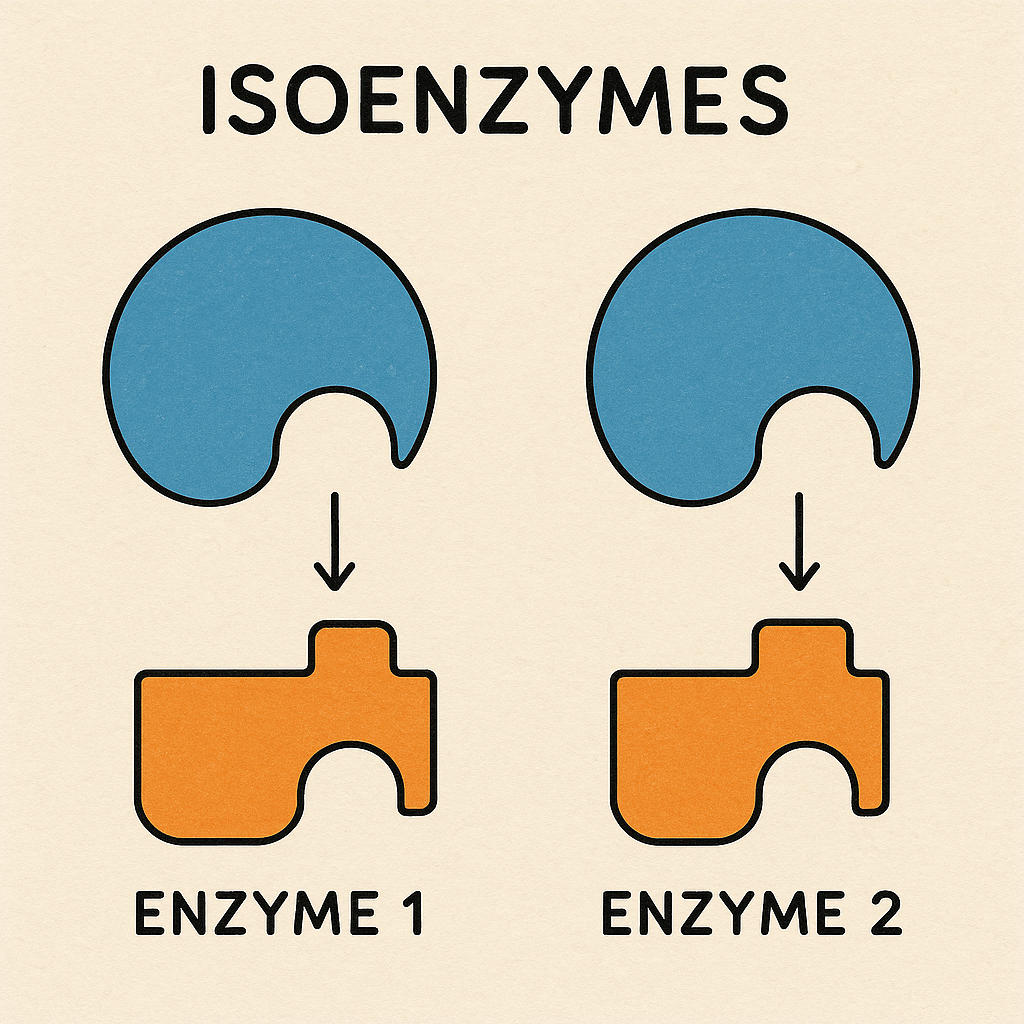
Definition of Isoenzymes
Isoenzymes (also called isozymes) are different molecular forms of the same enzyme that catalyze the same biochemical reaction but have distinct structural, electrophoretic, and kinetic properties. They exist in different tissues or cellular compartments and are encoded by different genes.
Characteristics and Properties of Isoenzymes
| Property | Description |
|---|---|
| Same Function | All isoenzymes catalyze the same reaction. |
| Different Structure | They have variations in amino acid sequences and subunit composition. |
| Tissue-Specific | Different isoenzymes are present in specific organs or tissues. |
| Varying Kinetics | Each isoenzyme has different enzyme kinetics (Km and Vmax values). |
| Genetic Origin | Encoded by different genes but evolved from a common ancestral gene. |
| Electrophoretic Mobility | Isoenzymes show different movement in electrophoresis due to charge differences. |
| Regulatory Properties | Some isoenzymes are regulated differently by activators or inhibitors. |
Types of Clinically Important Isoenzymes
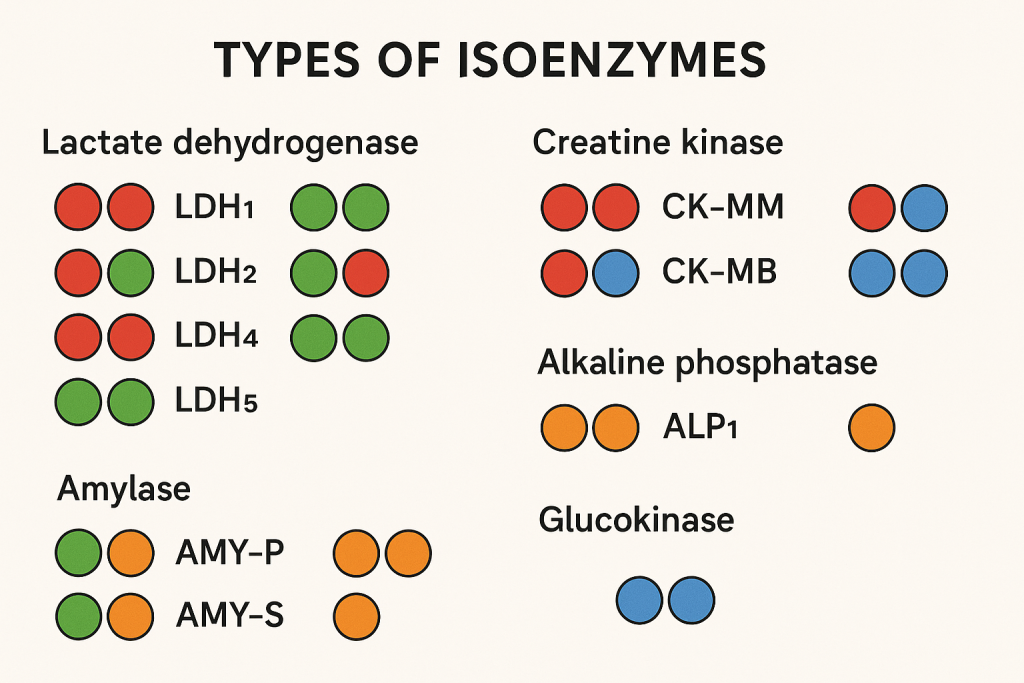
1. Lactate Dehydrogenase (LDH) Isoenzymes
LDH catalyzes the conversion of pyruvate to lactate. It has five isoforms composed of different combinations of H (Heart) and M (Muscle) subunits.
| LDH Isoenzyme | Composition (Subunits) | Tissue Distribution | Clinical Significance |
|---|---|---|---|
| LDH-1 (H4) | 4 Heart subunits | Heart, RBCs, Kidney | Increased in myocardial infarction |
| LDH-2 (H3M1) | 3 Heart + 1 Muscle | Reticuloendothelial system (RBCs, WBCs) | Increased in hemolysis |
| LDH-3 (H2M2) | 2 Heart + 2 Muscle | Lungs, Platelets | Increased in pulmonary infarction |
| LDH-4 (H1M3) | 1 Heart + 3 Muscle | Kidney, Pancreas | Increased in kidney damage |
| LDH-5 (M4) | 4 Muscle subunits | Liver, Skeletal Muscle | Increased in liver disease, muscle trauma |
Clinical Use: In heart attack, LDH-1 > LDH-2 (LDH flip) is a diagnostic indicator.
2. Creatine Kinase (CK) Isoenzymes
Creatine kinase catalyzes the conversion of creatine to phosphocreatine, supplying energy to muscles.
| CK Isoenzyme | Composition | Tissue Distribution | Clinical Significance |
|---|---|---|---|
| CK-MM | 2 Muscle subunits | Skeletal Muscle | Increased in muscular dystrophy, rhabdomyolysis |
| CK-MB | 1 Muscle + 1 Brain | Heart Muscle | Specific marker for myocardial infarction |
| CK-BB | 2 Brain subunits | Brain, Smooth Muscle | Increased in stroke, brain injury |
Clinical Use: CK-MB rises 4-6 hours after a heart attack, peaks at 12-24 hours, and normalizes within 2-3 days.
3. Alkaline Phosphatase (ALP) Isoenzymes
ALP catalyzes the hydrolysis of phosphate esters.
| ALP Isoenzyme | Tissue Distribution | Clinical Significance |
|---|---|---|
| Liver ALP | Liver | Increased in liver diseases (hepatitis, obstructive jaundice) |
| Bone ALP | Osteoblasts (Bone) | Elevated in rickets, osteomalacia, Paget’s disease |
| Placental ALP | Placenta | Increased in pregnancy, ovarian cancer |
| Intestinal ALP | Intestinal lining | Increased in inflammatory bowel disease |
Clinical Use: ALP isoenzyme differentiation helps in diagnosing bone vs. liver diseases.
4. Amylase Isoenzymes
Amylase breaks down starch into sugars.
| Amylase Isoenzyme | Tissue Distribution | Clinical Significance |
|---|---|---|
| Pancreatic Amylase (P-type) | Pancreas | Increased in acute pancreatitis |
| Salivary Amylase (S-type) | Salivary glands | Increased in mumps, salivary gland disorders |
Clinical Use: Pancreatic amylase is more specific for pancreatitis.
5. Gamma-Glutamyl Transferase (GGT) Isoenzymes
- Found in liver, kidney, pancreas
- Increased in alcohol-related liver disease
6. Acid Phosphatase (ACP) Isoenzymes
- Found in prostate, liver, spleen, RBCs
- Prostatic ACP increases in prostate cancer
Clinical Significance of Isoenzymes
1. Diagnosis of Myocardial Infarction (Heart Attack)
- CK-MB: Rises in 4-6 hours, peaks in 24 hours.
- LDH-1/LDH-2 Flip: LDH-1 becomes higher than LDH-2 in MI.
2. Liver and Bone Disease
- ALP Isoenzymes distinguish between liver and bone disorders.
- GGT is specific for liver damage.
3. Muscle and Neurological Disorders
- CK-MM: Elevated in muscular dystrophy.
- CK-BB: Increased in brain trauma, stroke.
4. Pancreatic Disorders
- Pancreatic amylase increases in acute pancreatitis.
5. Cancer Diagnosis
- Prostatic ACP: Elevated in prostate cancer.
- Placental ALP: Marker for ovarian and testicular tumors.
Laboratory Methods for Isoenzyme Analysis
- Electrophoresis (Most common) – Separates isoenzymes based on charge.
- Heat Inactivation Test – Distinguishes bone vs. liver ALP (bone ALP is heat-labile).
- Immunoassays – Antibody-based detection of specific isoenzymes.
- Chromatography (HPLC) – High-resolution separation.
Enzymes of Diagnostic Importance in Liver Diseases
Introduction
The liver plays a crucial role in metabolism, detoxification, and protein synthesis. Enzyme levels in the blood serve as biomarkers for liver function and damage. Liver enzymes are released into circulation due to hepatocellular injury, cholestasis, or metabolic dysfunction.
This article discusses clinically important liver enzymes, their normal ranges, and their clinical significance in different liver diseases.
1. Classification of Liver Enzymes
Liver enzymes can be classified into three major groups based on their diagnostic role:
A. Hepatocellular Injury Markers (Liver Cell Damage)
- Alanine Aminotransferase (ALT/SGPT)
- Aspartate Aminotransferase (AST/SGOT)
B. Cholestatic Markers (Bile Flow Obstruction)
- Alkaline Phosphatase (ALP)
- Gamma-Glutamyl Transferase (GGT)
C. Liver Function Assessment Enzymes
- Lactate Dehydrogenase (LDH)
- Glutamate Dehydrogenase (GLDH)
- 5′-Nucleotidase (5′-NT)
- Cholinesterase (CHE)
2. Hepatocellular Injury Markers
A. Alanine Aminotransferase (ALT/SGPT)
- Normal Range: 7-56 U/L
- Location: Found in the cytoplasm of hepatocytes
- Function: Converts alanine to pyruvate
- Clinical Significance:
- Highly specific for liver injury
- Markedly elevated in acute hepatitis (Viral, Alcoholic, Drug-induced, Autoimmune Hepatitis)
- Mildly elevated in fatty liver disease, cirrhosis
ALT is a more specific marker for liver damage than AST.
B. Aspartate Aminotransferase (AST/SGOT)
- Normal Range: 10-40 U/L
- Location: Found in the cytoplasm and mitochondria of liver, heart, muscle
- Function: Converts aspartate to oxaloacetate
- Clinical Significance:
- Elevated in both liver and non-liver conditions
- AST/ALT ratio >2: Suggests alcoholic liver disease
- AST/ALT ratio <1: Seen in non-alcoholic fatty liver disease (NAFLD)
- Increased in cirrhosis, viral hepatitis, and liver metastases
AST is less specific for liver disease because it is also found in the heart and muscles.
3. Cholestatic Markers (Bile Duct Obstruction)
A. Alkaline Phosphatase (ALP)
- Normal Range: 44-147 U/L
- Location: Found in bile ducts, bone, intestines, placenta
- Function: Hydrolyzes phosphate esters
- Clinical Significance:
- Markedly elevated in biliary obstruction (Gallstones, Primary Biliary Cirrhosis, Primary Sclerosing Cholangitis)
- Mildly increased in liver metastases, cirrhosis, fatty liver
- Increased in bone diseases (Paget’s disease, rickets, fractures)
ALP levels should be interpreted along with GGT to confirm liver involvement.
B. Gamma-Glutamyl Transferase (GGT)
- Normal Range: 8-61 U/L
- Location: Present in liver, pancreas, kidney
- Function: Transfers glutamyl groups in amino acid metabolism
- Clinical Significance:
- Highly sensitive for detecting alcohol-induced liver disease
- Markedly elevated in cholestasis, biliary obstruction, and liver tumors
- Helps differentiate liver from bone disease (If ALP is high but GGT is normal, it’s likely a bone disorder.)
GGT is more sensitive than ALP for liver damage caused by alcohol or drugs.
4. Liver Function Assessment Enzymes
A. Lactate Dehydrogenase (LDH)
- Normal Range: 140-280 U/L
- Location: Present in liver, heart, RBCs, kidneys, muscles
- Function: Converts pyruvate to lactate
- Clinical Significance:
- Increased in hepatic ischemia, liver metastases, and hemolysis
- LDH-5 isoenzyme is liver-specific
LDH is less specific for liver disease and must be used with other markers.
B. Glutamate Dehydrogenase (GLDH)
- Normal Range: 0-8 U/L
- Location: Mitochondria of hepatocytes
- Function: Catalyzes oxidative deamination of glutamate
- Clinical Significance:
- Highly specific for severe liver damage (fulminant hepatitis, liver failure)
- Increased in hepatocellular carcinoma (HCC)
C. 5′-Nucleotidase (5′-NT)
- Normal Range: 2-17 U/L
- Location: Plasma membrane of liver cells
- Function: Hydrolyzes nucleotides
- Clinical Significance:
- Elevated in cholestasis, primary biliary cirrhosis
- More specific than ALP for liver diseases
D. Cholinesterase (CHE)
- Normal Range: 5000-12000 U/L
- Location: Synthesized by the liver
- Function: Hydrolyzes choline esters (acetylcholine)
- Clinical Significance:
- Low levels indicate severe liver dysfunction (cirrhosis, liver failure)
- Used to assess prognosis in liver disease
5. Clinical Interpretation of Liver Enzyme Patterns
| Enzyme Pattern | Possible Liver Condition |
|---|---|
| ALT ↑↑, AST ↑ (AST/ALT < 1) | Viral Hepatitis, Non-Alcoholic Fatty Liver Disease (NAFLD) |
| AST ↑↑, ALT ↑ (AST/ALT > 2) | Alcoholic Liver Disease |
| ALP ↑↑, GGT ↑↑ | Cholestasis, Biliary Obstruction |
| ALP ↑, GGT Normal | Bone Disease |
| LDH-5 ↑ | Liver Metastases, Hepatic Ischemia |
| Cholinesterase ↓ | Severe Liver Failure |
6. Liver Enzyme Elevation Causes
A. Mild Elevation (<2x Normal)
- Fatty liver disease (NAFLD)
- Medications (Statins, NSAIDs)
- Alcohol consumption
- Chronic hepatitis
B. Moderate Elevation (2-5x Normal)
- Viral hepatitis (Hepatitis B, C)
- Autoimmune hepatitis
- Cirrhosis
- Liver metastases
C. Severe Elevation (>5x Normal)
- Acute hepatitis (Viral, Drug-induced)
- Liver failure
- Hepatic ischemia
- Toxin exposure (Paracetamol overdose).
Comprehensive Overview of ALT, AST, ALP, and GGT in Liver Diseases
Liver function tests (LFTs) include Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Alkaline Phosphatase (ALP), and Gamma-Glutamyl Transferase (GGT), which help diagnose hepatocellular damage, cholestasis, and metabolic liver disorders.
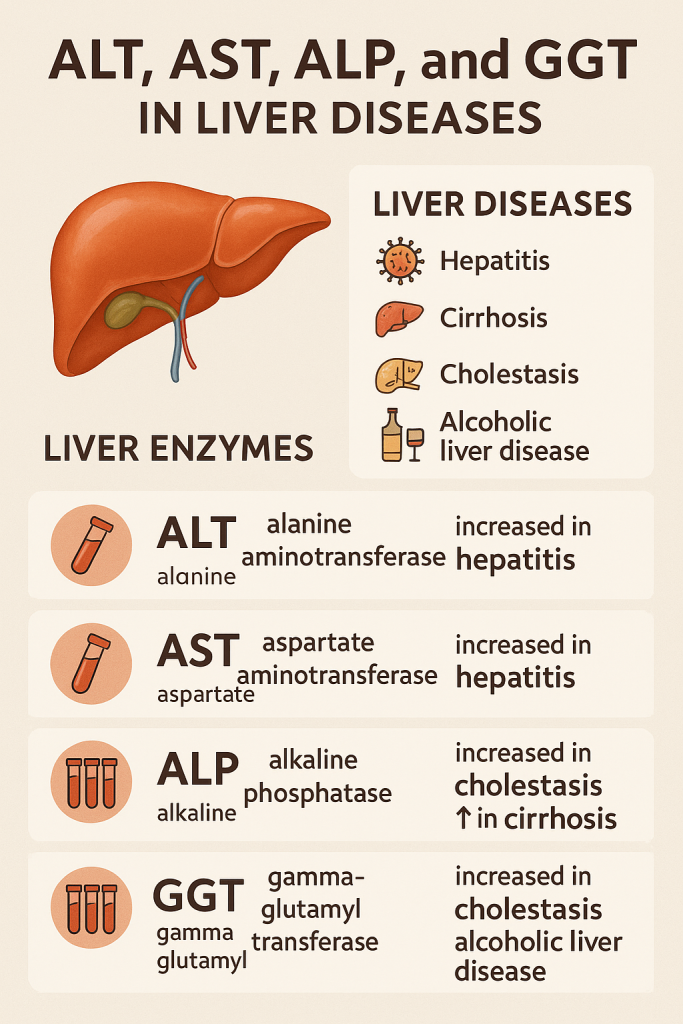
1. Alanine Aminotransferase (ALT / SGPT)
Definition:
- ALT (SGPT) is an enzyme found primarily in the liver and is specific for liver injury.
- It catalyzes the conversion of alanine to pyruvate, aiding in gluconeogenesis.
Normal Range:
- 7-56 U/L (May vary slightly between laboratories)
Tissue Distribution:
- Liver (Highest concentration)
- Kidney
- Muscle
Clinical Significance:
- ALT is highly specific for hepatocellular damage because it is mainly found in the liver.
- Markedly increased in:
- Acute viral hepatitis (Hepatitis A, B, C, D, E)
- Toxic liver injury (Drugs, Alcohol, Paracetamol overdose)
- Ischemic hepatitis (Liver hypoxia due to shock)
- Mild to moderate increase in:
- Non-Alcoholic Fatty Liver Disease (NAFLD)
- Cirrhosis
- Chronic hepatitis
- Liver metastases
ALT is more liver-specific than AST and is used to detect early liver damage.
2. Aspartate Aminotransferase (AST / SGOT)
Definition:
- AST (SGOT) is an enzyme found in the liver, heart, muscle, kidney, and brain.
- It catalyzes the conversion of aspartate to oxaloacetate, playing a role in the Krebs cycle.
Normal Range:
- 10-40 U/L
Tissue Distribution:
- Liver
- Heart (Myocardium)
- Skeletal Muscle
- Kidney, Brain, Pancreas, RBCs
Clinical Significance:
- Increased in liver and non-liver conditions due to its wide tissue distribution.
- Markedly increased in:
- Acute hepatitis
- Liver cirrhosis
- Liver metastases
- Myocardial infarction (Heart attack)
- Rhabdomyolysis (Muscle breakdown)
- AST/ALT Ratio Interpretation:
- AST/ALT > 2: Suggests Alcoholic Liver Disease
- AST/ALT < 1: Suggests Non-Alcoholic Fatty Liver Disease (NAFLD)
AST is less specific for liver disease than ALT because it is also found in muscles and the heart.
3. Alkaline Phosphatase (ALP)
Definition:
- ALP is an enzyme involved in dephosphorylation reactions and is important for bone and bile metabolism.
Normal Range:
- 44-147 U/L
Tissue Distribution:
- Liver (Biliary tract)
- Bone (Osteoblasts)
- Placenta (During pregnancy)
- Intestines, Kidneys
Clinical Significance:
- Markedly increased in:
- Cholestasis (Biliary obstruction due to Gallstones, Primary Biliary Cirrhosis, Primary Sclerosing Cholangitis)
- Liver metastases or infiltrative liver disease
- Bone diseases (Rickets, Paget’s disease, Osteomalacia, Bone fractures)
- Mild increase in:
- Fatty liver disease (NAFLD)
- Hepatitis
- Liver cirrhosis
ALP vs. Bone Disease:
- If ALP is high, check GGT.
- ALP ↑ & GGT ↑ → Liver origin (Cholestasis, Liver disease)
- ALP ↑ & GGT Normal → Bone disease
4. Gamma-Glutamyl Transferase (GGT)
Definition:
- GGT is an enzyme that catalyzes the transfer of the γ-glutamyl group from peptides to amino acids.
- It is highly sensitive for detecting liver damage caused by alcohol and drugs.
Normal Range:
- 8-61 U/L
Tissue Distribution:
- Liver (Highest concentration)
- Pancreas
- Kidney
- Intestines
Clinical Significance:
- Highly increased in:
- Alcoholic liver disease
- Cholestasis (Biliary obstruction)
- Liver tumors, metastases
- Pancreatitis
- Used to differentiate ALP elevations:
- If ALP ↑ & GGT ↑ → Liver disease (Biliary obstruction)
- If ALP ↑ & GGT Normal → Bone disease
- Most sensitive marker for detecting chronic alcohol consumption.
GGT is an early marker of liver injury due to alcohol and drugs.
5. Clinical Interpretation of Liver Enzymes
| Enzyme Pattern | Possible Liver Disease |
|---|---|
| ALT ↑↑, AST ↑ (AST/ALT < 1) | Viral Hepatitis, NAFLD |
| AST ↑↑, ALT ↑ (AST/ALT > 2) | Alcoholic Liver Disease |
| ALP ↑↑, GGT ↑↑ | Cholestasis, Biliary Obstruction |
| ALP ↑, GGT Normal | Bone Disease (Paget’s, Rickets, Fractures) |
| GGT ↑↑ with AST/ALT Normal | Alcoholic Liver Disease, Drug-Induced Liver Injury |
| LDH-5 ↑ | Liver Metastases, Hepatic Ischemia |
6. Liver Enzyme Elevation Causes
A. Mild Elevation (<2x Normal)
- Fatty Liver Disease (NAFLD)
- Chronic Hepatitis
- Alcohol Consumption
- Drug-Induced Liver Injury (Statins, NSAIDs)
B. Moderate Elevation (2-5x Normal)
- Acute Hepatitis (Viral, Alcoholic, Drug-Induced)
- Liver Cirrhosis
- Liver Metastases
C. Severe Elevation (>5x Normal)
- Acute Hepatitis (Hepatitis A, B, C)
- Toxin-Induced Liver Failure (Paracetamol Overdose, Poisoning)
- Hepatic Ischemia (Shock Liver)
7. Summary & Key Points
- ALT (SGPT): Most liver-specific, increased in viral hepatitis & fatty liver disease.
- AST (SGOT): Found in liver, heart, muscle, increased in alcoholic liver disease.
- ALP: Elevated in biliary obstruction & bone disease.
- GGT: Sensitive for alcohol-related liver damage & biliary obstruction.
Comprehensive Overview of Myocardial Infarction (Heart Attack) and Diagnostic Enzymes
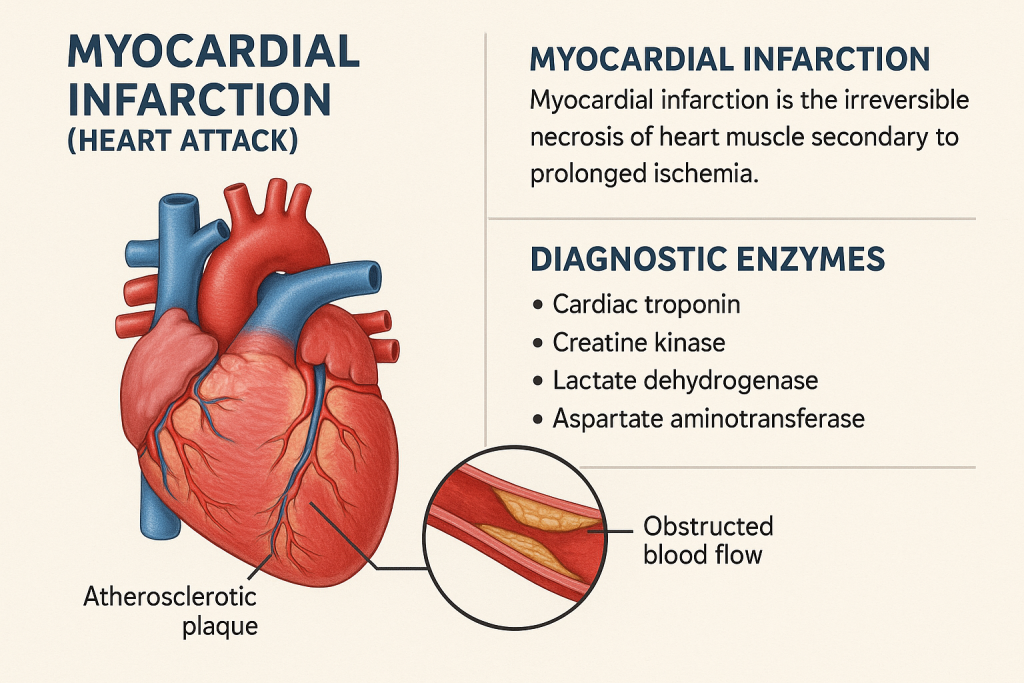
Introduction
A Myocardial Infarction (MI), commonly known as a heart attack, occurs due to blockage of coronary arteries, leading to ischemia and necrosis of heart muscle (myocardium). Diagnosis is based on clinical symptoms, ECG changes, and specific cardiac biomarkers.
This article discusses cardiac enzymes and biomarkers, their normal ranges, and their clinical significance in MI diagnosis and monitoring.
1. Classification of Cardiac Biomarkers
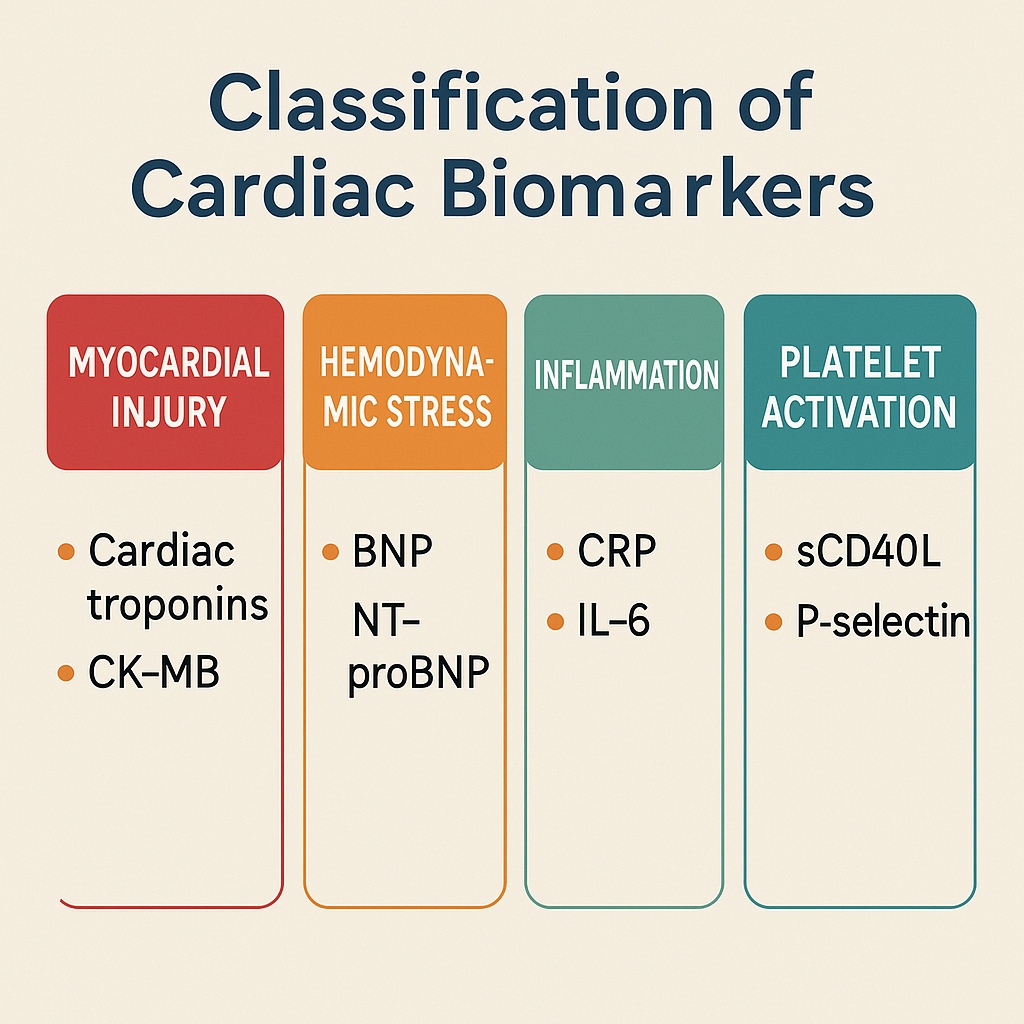
Cardiac biomarkers are classified into three groups:
A. Early Markers (Detected within 1-6 hours of MI)
- Myoglobin
- Creatine Kinase-MB (CK-MB)
- Troponin I & T (Highly Sensitive Troponins – hs-cTnI, hs-cTnT)
B. Intermediate Markers (6-24 hours post-MI)
- Creatine Kinase (CK-MB)
- Troponins (TnI, TnT)
- Lactate Dehydrogenase (LDH-1 & LDH-2)
C. Late Markers (24-72 hours post-MI)
- Troponins (Remain elevated for 7-14 days)
- LDH (Peaks at 48-72 hours, normalizes in 10 days)
2. Creatine Kinase-MB (CK-MB)
Definition:
- CK-MB is a cardiac-specific isoenzyme of Creatine Kinase (CK).
- It catalyzes ATP-dependent phosphorylation of creatine, providing energy to cardiac muscles.
Normal Range:
- 0-5 ng/mL or <6% of total CK
Tissue Distribution:
- Heart Muscle (Most specific)
- Skeletal Muscle (Minimal)
Clinical Significance:
- Highly specific for myocardial infarction (MI)
- Rises: 4-6 hours after MI
- Peaks: 12-24 hours
- Returns to normal: 48-72 hours
CK-MB vs. Total CK:
- CK-MB/Total CK ratio >6% suggests cardiac origin.
- CK-MB/Total CK ratio <3% suggests skeletal muscle injury.
CK-MB is useful for detecting reinfarction (Second heart attack within a few days).
3. Troponins (cTnI & cTnT)
Definition:
- Troponins are cardiac-specific proteins that regulate muscle contraction.
- Troponin I (cTnI) and Troponin T (cTnT) are highly specific to cardiac muscle.
Normal Range:
- Troponin I (cTnI): <0.04 ng/mL
- Troponin T (cTnT): <0.01 ng/mL
- High-Sensitivity Troponins (hs-cTnI, hs-cTnT): Detect ultra-low levels, improving early MI detection.
Tissue Distribution:
- Heart Muscle (Cardiac Specific)
- Not found in skeletal muscle
Clinical Significance:
- Most specific and sensitive biomarker for myocardial infarction.
- Rises: 3-6 hours after MI
- Peaks: 12-24 hours
- Remains elevated: 7-14 days
- High-Sensitivity Troponins (hs-cTn): Detect very small MI events even before symptoms.
Troponins are the gold standard for MI diagnosis due to their high specificity.
4. Myoglobin
Definition:
- Myoglobin is a small oxygen-binding protein found in muscle cells.
- It is released quickly from damaged cardiac or skeletal muscle.
Normal Range:
- <85 ng/mL
Tissue Distribution:
- Skeletal Muscle
- Cardiac Muscle
Clinical Significance:
- Earliest marker of MI (Rises within 1-2 hours).
- Rises: 1-2 hours after MI
- Peaks: 6-9 hours
- Returns to normal: 24 hours
- High sensitivity, but low specificity (Also elevated in muscle injuries, rhabdomyolysis).
Myoglobin is useful for early detection of MI but must be confirmed with Troponins.
5. Lactate Dehydrogenase (LDH)
Definition:
- LDH catalyzes the conversion of lactate to pyruvate and is found in many tissues.
- LDH-1 (Heart) and LDH-2 (Liver, RBCs) are important in MI.
Normal Range:
- 125-250 U/L
- LDH-1/LDH-2 Ratio: Normally LDH-2 > LDH-1.
Tissue Distribution:
- LDH-1: Heart, RBCs
- LDH-2: Liver, Kidney, WBCs
- LDH-3: Lungs, Spleen
- LDH-4: Pancreas
- LDH-5: Liver, Skeletal Muscle
Clinical Significance:
- Late marker of MI
- Rises: 6-12 hours after MI
- Peaks: 48-72 hours
- Returns to normal: 10-14 days
- LDH-1/LDH-2 Flip: LDH-1 > LDH-2 indicates MI.
LDH is not specific to MI but useful for late-stage detection.
6. Cardiac Enzyme Timeline in Myocardial Infarction
| Marker | Rises (Hours) | Peaks (Hours) | Returns to Normal (Days) | Specificity |
|---|---|---|---|---|
| Myoglobin | 1-2 | 6-9 | 1 | Low (Muscle injuries) |
| CK-MB | 4-6 | 12-24 | 2-3 | High |
| Troponin I (cTnI) | 3-6 | 12-24 | 7-14 | Very High |
| Troponin T (cTnT) | 3-6 | 12-24 | 7-14 | Very High |
| LDH-1 | 6-12 | 48-72 | 10-14 | Moderate |
7. Clinical Interpretation of Cardiac Enzymes
| Enzyme Pattern | Possible Diagnosis |
|---|---|
| CK-MB ↑, Troponin ↑ | Acute Myocardial Infarction (MI) |
| Troponin ↑, CK-MB Normal | Small MI, Chronic Cardiac Injury |
| LDH-1/LDH-2 Flip (LDH-1 > LDH-2) | Late-stage MI |
| Myoglobin ↑, CK-MB Normal | Skeletal Muscle Injury |
8. Causes of Cardiac Enzyme Elevation
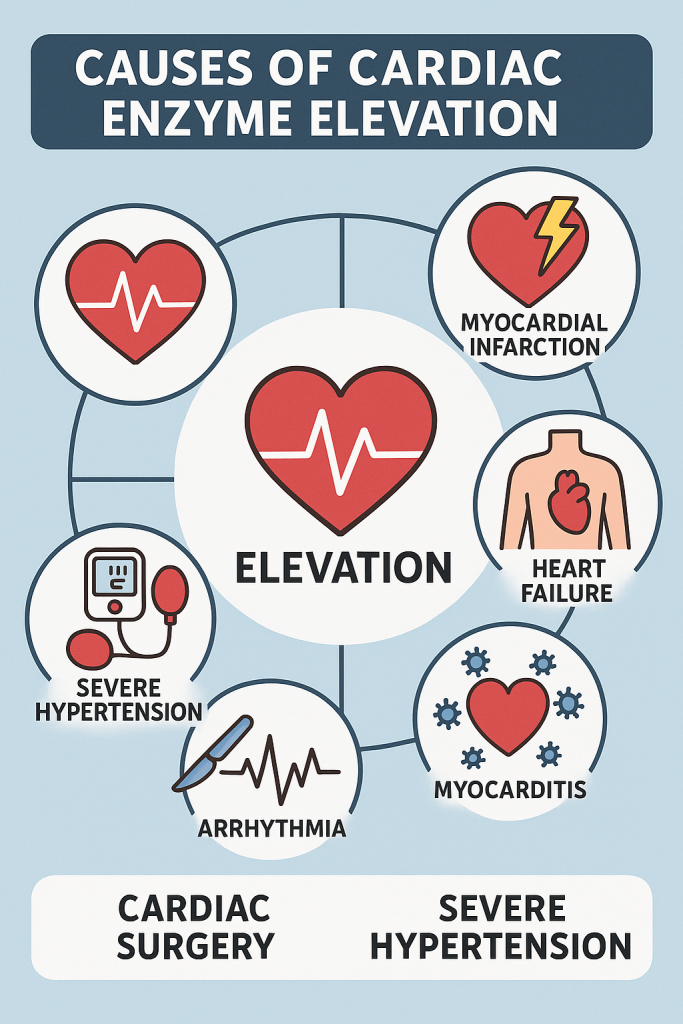
A. Cardiac Causes
- Myocardial Infarction (STEMI, NSTEMI)
- Myocarditis (Heart inflammation)
- Heart Failure
- Cardiac Trauma (Surgery, Injury)
B. Non-Cardiac Causes
- Rhabdomyolysis (Skeletal Muscle Breakdown)
- Pulmonary Embolism
- Sepsis, Shock
- Renal Failure (Delayed Troponin clearance).
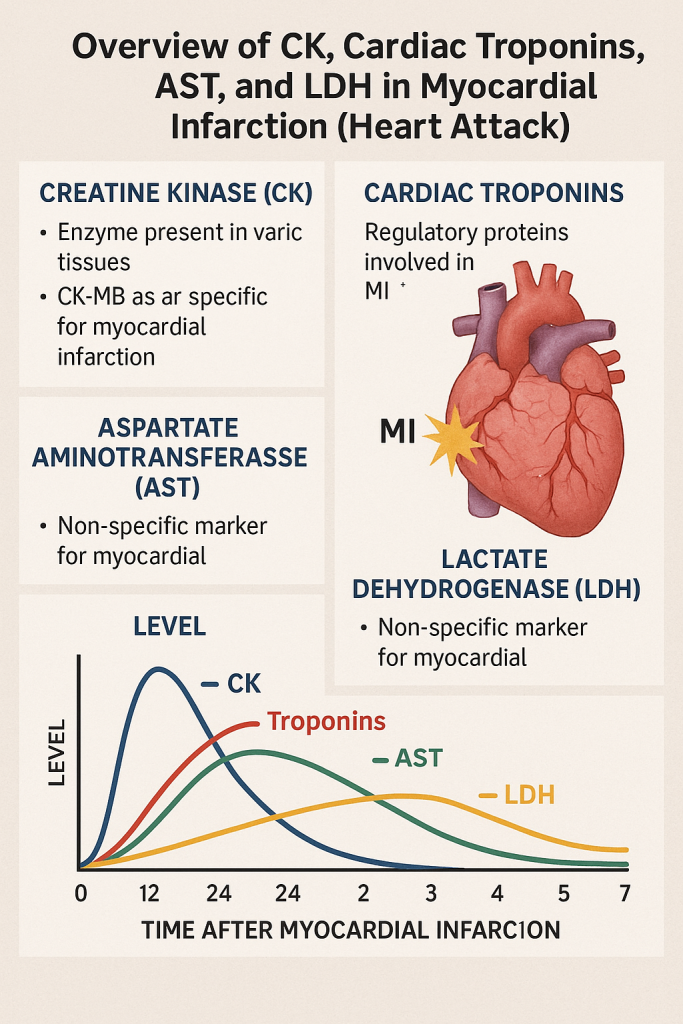
Comprehensive Overview of CK, Cardiac Troponins, AST, and LDH in Myocardial Infarction (Heart Attack)
Introduction
Myocardial Infarction (MI, or Heart Attack) occurs when there is ischemia and necrosis of heart muscle due to blockage of coronary arteries. Diagnosis is based on ECG changes, clinical symptoms, and cardiac biomarkers.
This article provides a detailed analysis of the key enzymes and biomarkers used in MI diagnosis:
- Creatine Kinase (CK & CK-MB)
- Cardiac Troponins (cTnI & cTnT)
- Aspartate Aminotransferase (AST)
- Lactate Dehydrogenase (LDH)
1. Creatine Kinase (CK) and CK-MB
Definition:
- Creatine Kinase (CK) is an enzyme that catalyzes the conversion of creatine to phosphocreatine, providing energy for muscle contraction.
- CK has three isoenzymes:
- CK-MM (Skeletal Muscle)
- CK-MB (Cardiac Muscle – Myocardium)
- CK-BB (Brain & Smooth Muscle)
Normal Range:
- Total CK: 20-200 U/L
- CK-MB: 0-5 ng/mL (<6% of total CK)
Tissue Distribution:
- CK-MB is predominantly in the heart muscle, making it a key marker for MI.
Clinical Significance:
- CK-MB Rises: 4-6 hours after MI
- CK-MB Peaks: 12-24 hours
- CK-MB Normalizes: 48-72 hours
CK-MB vs. Total CK Ratio Interpretation:
- CK-MB/Total CK >6% → Cardiac origin (MI likely)
- CK-MB/Total CK <3% → Skeletal muscle injury
CK-MB is useful for detecting reinfarction (a second heart attack occurring within a few days).
2. Cardiac Troponins (cTnI & cTnT)
Definition:
- Cardiac Troponins (cTnI & cTnT) are proteins that regulate muscle contraction in the heart.
- Troponin I (cTnI) and Troponin T (cTnT) are specific to the heart, making them gold-standard biomarkers for MI.
- High-Sensitivity Troponins (hs-cTnI, hs-cTnT) detect even very small heart injuries.
Normal Range:
- Troponin I (cTnI): <0.04 ng/mL
- Troponin T (cTnT): <0.01 ng/mL
Tissue Distribution:
- Only in Cardiac Muscle (Not found in skeletal muscle)
Clinical Significance:
- Most specific and sensitive biomarker for myocardial infarction (MI).
- Troponins Rise: 3-6 hours after MI
- Troponins Peak: 12-24 hours
- Troponins Remain Elevated: 7-14 days
- High-Sensitivity Troponins (hs-cTnI & hs-cTnT):
- Detect ultra-small MI events even before symptoms appear.
- Used in early detection of unstable angina and silent MI.
Troponins are the gold standard for diagnosing MI due to their high specificity and long duration in the blood.
3. Aspartate Aminotransferase (AST / SGOT)
Definition:
- AST (SGOT) is an enzyme found in the heart, liver, skeletal muscle, and kidneys.
- It catalyzes the conversion of aspartate to oxaloacetate, playing a role in the Krebs cycle.
Normal Range:
- 10-40 U/L
Tissue Distribution:
- Heart (Myocardium)
- Liver
- Skeletal Muscle
- Kidney, Brain, Pancreas, RBCs
Clinical Significance in MI:
- Rises: 6-12 hours after MI
- Peaks: 24-48 hours
- Returns to normal: 3-5 days
- AST/ALT Ratio:
- AST > ALT in MI (Unlike in liver disease where ALT > AST)
- AST is less specific because it is also found in the liver and muscles.
AST is a supportive biomarker but not as specific for MI as Troponins or CK-MB.
4. Lactate Dehydrogenase (LDH)
Definition:
- LDH catalyzes the conversion of lactate to pyruvate and is found in various tissues.
- LDH-1 and LDH-2 are the most relevant in heart disease.
Normal Range:
- 125-250 U/L
- LDH-1/LDH-2 Ratio:
- Normally, LDH-2 > LDH-1
- LDH-1/LDH-2 Flip (LDH-1 > LDH-2) → Indicates Myocardial Infarction
Tissue Distribution:
- LDH-1: Heart, RBCs
- LDH-2: Liver, Kidney, WBCs
- LDH-3: Lungs, Spleen
- LDH-4: Pancreas
- LDH-5: Liver, Skeletal Muscle
Clinical Significance:
- Rises: 6-12 hours after MI
- Peaks: 48-72 hours
- Returns to normal: 10-14 days
- LDH-1/LDH-2 Flip:
- Normally LDH-2 > LDH-1
- If LDH-1 > LDH-2, it suggests Myocardial Infarction.
LDH is useful for detecting late-stage MI when CK-MB and Troponins have normalized.
5. Timeline of Cardiac Enzyme Changes in Myocardial Infarction
| Marker | Rises (Hours) | Peaks (Hours) | Returns to Normal (Days) | Specificity |
|---|---|---|---|---|
| Myoglobin | 1-2 | 6-9 | 1 | Low (Muscle injuries) |
| CK-MB | 4-6 | 12-24 | 2-3 | High |
| Troponin I (cTnI) | 3-6 | 12-24 | 7-14 | Very High |
| Troponin T (cTnT) | 3-6 | 12-24 | 7-14 | Very High |
| AST | 6-12 | 24-48 | 3-5 | Moderate |
| LDH-1 | 6-12 | 48-72 | 10-14 | Moderate |
6. Clinical Interpretation of Cardiac Enzymes
| Enzyme Pattern | Possible Diagnosis |
|---|---|
| CK-MB ↑, Troponin ↑ | Acute Myocardial Infarction (MI) |
| Troponin ↑, CK-MB Normal | Small MI, Chronic Cardiac Injury |
| LDH-1/LDH-2 Flip (LDH-1 > LDH-2) | Late-stage MI |
| AST ↑, CK-MB Normal | Skeletal Muscle Injury or Liver Disease |
Comprehensive Overview of Muscle Diseases and Diagnostic Enzymes
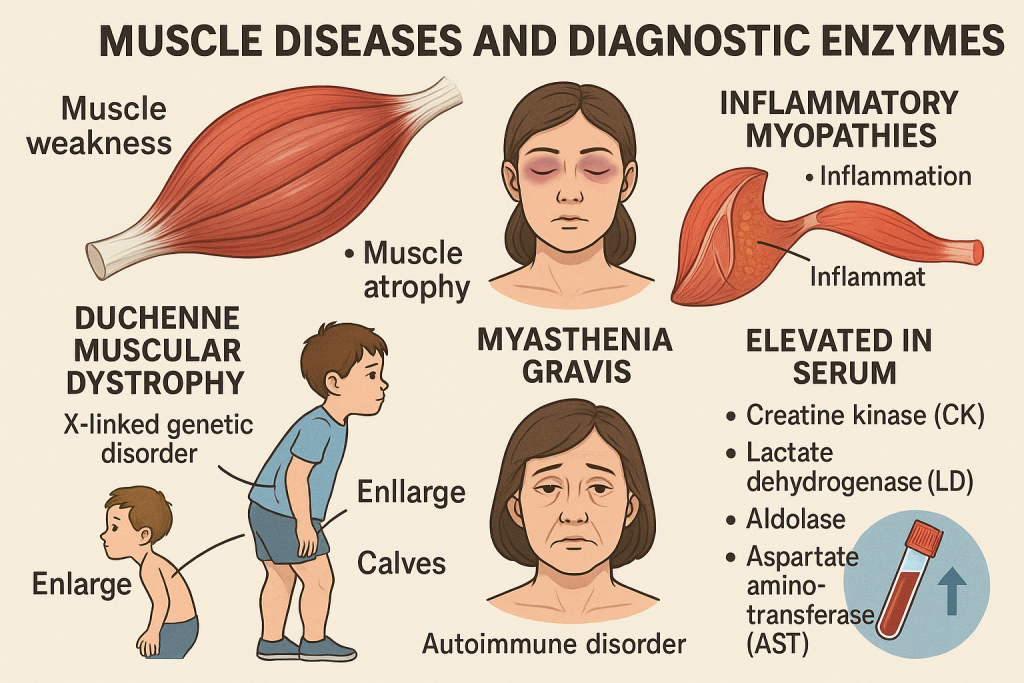
Introduction
Muscle diseases (myopathies) include a broad spectrum of disorders affecting skeletal, cardiac, and smooth muscles. They can be classified into:
- Primary Muscle Diseases (Inherited, Acquired, Metabolic)
- Secondary Muscle Diseases (Autoimmune, Neurological, Endocrine)
Laboratory enzymes and biomarkers play a crucial role in diagnosing, monitoring, and differentiating muscle diseases.
1. Important Enzymes in Muscle Diseases
| Enzyme | Primary Tissue Location | Function | Clinical Significance |
|---|---|---|---|
| Creatine Kinase (CK/CPK) | Skeletal & Cardiac Muscle | Energy metabolism | ↑ in muscle damage (DMD, Polymyositis) |
| Lactate Dehydrogenase (LDH) | Muscle, RBCs, Liver | Anaerobic metabolism | ↑ in muscle necrosis, rhabdomyolysis |
| Aspartate Aminotransferase (AST) | Muscle, Liver, Heart | Amino acid metabolism | ↑ in muscle diseases, liver disorders |
| Aldolase | Skeletal Muscle | Glycolysis enzyme | ↑ in muscular dystrophies, inflammatory myopathies |
| Myoglobin | Muscle | Oxygen transport | ↑ in rhabdomyolysis, muscle trauma |
2. Creatine Kinase (CK/CPK) in Muscle Diseases
Definition:
- Creatine Kinase (CK/CPK) is an enzyme involved in energy metabolism in muscle cells.
- CK has three isoenzymes:
- CK-MM (Skeletal muscle-specific)
- CK-MB (Cardiac muscle-specific)
- CK-BB (Brain and smooth muscle)
Normal Range:
- Total CK: 20-200 U/L
- CK-MM: 98% of total CK
- CK-MB: <5% of total CK
- CK-BB: Minimal in serum
Clinical Significance:
- CK is the most important marker of muscle damage.
- CK-MM ↑ in skeletal muscle diseases:
- Duchenne Muscular Dystrophy (DMD)
- Becker Muscular Dystrophy (BMD)
- Polymyositis & Dermatomyositis
- Rhabdomyolysis
- Intensive exercise, trauma
CK in Duchenne Muscular Dystrophy (DMD):
- CK levels can be 50-100 times the normal range in early stages.
- CK decreases in later stages due to progressive muscle degeneration.
CK levels correlate with the severity of muscle disease.
3. Lactate Dehydrogenase (LDH) in Muscle Diseases
Definition:
- LDH catalyzes lactate to pyruvate during anaerobic metabolism.
- LDH-5 (Muscle-specific) is important in myopathies.
Normal Range:
- 125-250 U/L
Clinical Significance:
- LDH ↑ in muscle necrosis & damage
- Duchenne Muscular Dystrophy (DMD)
- Rhabdomyolysis
- Muscle trauma (Crush Injury)
- Polymyositis & Dermatomyositis
LDH-1 to LDH-5 in Muscle Diseases:
| LDH Isoenzyme | Primary Location | Clinical Use |
|---|---|---|
| LDH-1 | Heart, RBCs | MI, Hemolysis |
| LDH-2 | Heart, WBCs | MI, Hemolysis |
| LDH-3 | Lungs, Spleen | Pulmonary diseases |
| LDH-4 | Kidney, Pancreas | Renal disorders |
| LDH-5 | Skeletal Muscle, Liver | Muscle disease, Hepatic disorders |
LDH-5 is significantly elevated in muscle diseases.
4. Aspartate Aminotransferase (AST) in Muscle Diseases
Definition:
- AST (SGOT) is an enzyme involved in amino acid metabolism.
- It is present in the liver, heart, and skeletal muscle.
Normal Range:
- 10-40 U/L
Clinical Significance in Muscle Diseases:
- AST is elevated in:
- Muscular Dystrophies
- Rhabdomyolysis
- Polymyositis & Dermatomyositis
- Intensive exercise, trauma
AST is less specific than CK for muscle disease since it is also found in the liver.
5. Aldolase in Muscle Diseases
Definition:
- Aldolase is a glycolytic enzyme involved in energy production in muscle.
Normal Range:
- 1-7 U/L
Clinical Significance:
- Aldolase is elevated in:
- Muscular Dystrophies (DMD, BMD)
- Polymyositis & Dermatomyositis
- Metabolic Myopathies (Glycogen storage diseases)
Aldolase is useful in differentiating muscle vs. liver disease (not affected by liver disease).
6. Myoglobin in Muscle Diseases
Definition:
- Myoglobin is an oxygen-binding protein found in muscle cells.
Normal Range:
- <85 ng/mL
Clinical Significance:
- Myoglobin is the earliest marker of muscle damage.
- Rises: 1-2 hours after muscle injury
- Peaks: 6-9 hours
- Returns to normal: 24 hours
- Myoglobin is elevated in:
- Rhabdomyolysis (Muscle breakdown due to trauma, toxins, infections)
- Crush injuries, Burns
- Severe Exercise (Extreme Athletes)
High myoglobin levels can cause kidney damage (Myoglobinuria).
7. Muscle Enzyme Elevation in Different Myopathies
| Muscle Disease | CK | LDH | AST | Aldolase | Myoglobin |
|---|---|---|---|---|---|
| Duchenne Muscular Dystrophy (DMD) | ↑↑↑ | ↑ | ↑ | ↑ | Normal |
| Becker Muscular Dystrophy (BMD) | ↑ | ↑ | ↑ | ↑ | Normal |
| Polymyositis/Dermatomyositis | ↑↑ | ↑ | ↑ | ↑ | ↑ |
| Rhabdomyolysis | ↑↑↑ | ↑↑ | ↑↑ | Normal | ↑↑↑ |
| Crush Injury | ↑↑↑ | ↑↑ | ↑↑ | Normal | ↑↑↑ |
| Glycogen Storage Diseases | ↑ | ↑ | Normal | ↑ | Normal |
8. Clinical Interpretation of Muscle Enzymes
| Enzyme Pattern | Possible Diagnosis |
|---|---|
| CK-MM ↑, Aldolase ↑ | Muscular Dystrophy |
| CK-MM ↑↑↑, Myoglobin ↑↑↑ | Rhabdomyolysis |
| LDH-5 ↑, CK-MM ↑ | Polymyositis, Dermatomyositis |
| CK ↑, AST ↑, ALT Normal | Muscle Disease |
| CK Normal, Aldolase Normal | Neuromuscular Disorder (ALS, Myasthenia Gravis) |
Comprehensive Overview of Creatine Kinase (CK) and Aldolase in Muscle Diseases
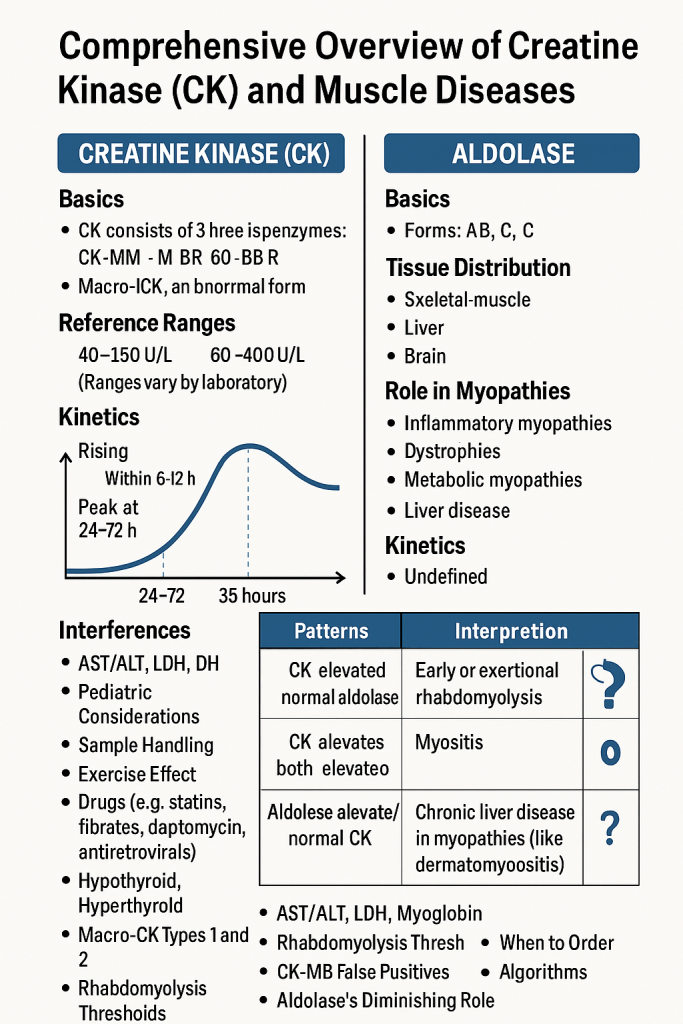
Introduction
Muscle diseases (myopathies) involve skeletal, cardiac, or smooth muscle damage due to genetic, inflammatory, metabolic, or traumatic causes. Creatine Kinase (CK) and Aldolase are two key muscle enzymes used in diagnosing and monitoring these conditions.
This article provides detailed insights into CK and Aldolase, including their normal ranges, tissue distribution, and clinical significance.
1. Creatine Kinase (CK/CPK) in Muscle Diseases
Definition:
- Creatine Kinase (CK or CPK) is an enzyme involved in energy metabolism in muscle cells.
- It catalyzes the phosphorylation of creatine, producing phosphocreatine, which serves as an energy reserve for muscle contraction.
Isoenzymes of CK:
| CK Isoenzyme | Primary Tissue Location | Clinical Significance |
|---|---|---|
| CK-MM (98% of total CK) | Skeletal muscle | ↑ in muscular dystrophies, inflammatory myopathies, rhabdomyolysis |
| CK-MB (<5% of total CK) | Cardiac muscle | ↑ in myocardial infarction (MI) |
| CK-BB (Minimal in serum) | Brain & smooth muscle | ↑ in brain trauma, stroke, malignancies |
Normal Range:
- Total CK: 20-200 U/L
- CK-MM: 98% of total CK
- CK-MB: <5% of total CK
- CK-BB: Minimal in serum
Tissue Distribution:
- Skeletal Muscle (CK-MM)
- Cardiac Muscle (CK-MB)
- Brain, Smooth Muscle (CK-BB)
Clinical Significance:
- CK-MM is the most important marker of skeletal muscle damage.
- CK-MM is markedly elevated in:
- Duchenne Muscular Dystrophy (DMD) (50-100 times normal in early stages)
- Becker Muscular Dystrophy (BMD)
- Rhabdomyolysis (Severe muscle breakdown)
- Polymyositis & Dermatomyositis
- Intensive exercise, muscle trauma
CK in Duchenne Muscular Dystrophy (DMD):
- CK levels are extremely high (50-100 times normal).
- CK decreases in later stages due to muscle fiber loss and replacement with fat & fibrosis.
CK in Rhabdomyolysis:
- Massive CK-MM elevation (>10,000 U/L)
- Accompanied by myoglobinuria (can cause acute kidney injury).
CK is the primary biomarker for muscle disease, correlating with the severity of damage.
2. Aldolase in Muscle Diseases
Definition:
- Aldolase is an enzyme involved in glycolysis, breaking down fructose-1,6-bisphosphate into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate.
- It plays a crucial role in muscle energy metabolism.
Normal Range:
- 1-7 U/L
Tissue Distribution:
- Skeletal Muscle
- Liver
- Brain
Clinical Significance:
- Aldolase is elevated in:
- Duchenne Muscular Dystrophy (DMD)
- Becker Muscular Dystrophy (BMD)
- Polymyositis & Dermatomyositis
- Metabolic Myopathies (Glycogen Storage Diseases, McArdle’s Disease)
- Normal Aldolase levels in neurogenic disorders (ALS, Myasthenia Gravis).
Aldolase is useful in differentiating primary muscle diseases from neurological disorders.
3. Comparison of CK and Aldolase in Muscle Diseases
| Feature | Creatine Kinase (CK) | Aldolase |
|---|---|---|
| Primary Function | Energy metabolism (Phosphocreatine system) | Glycolysis (Sugar breakdown) |
| Most Specific For | Muscle damage | Muscle energy metabolism |
| Normal Range | 20-200 U/L | 1-7 U/L |
| Elevated in | DMD, BMD, Rhabdomyolysis, Polymyositis, Trauma | DMD, BMD, Polymyositis, Metabolic Myopathies |
| Usefulness | Best for acute muscle damage | Useful in chronic myopathies |
| Effect of Liver Disease | Affected (Also found in the liver) | Not affected (Specific to muscle) |
Aldolase is more useful in inflammatory and metabolic myopathies, while CK is the best marker for acute muscle damage.
4. Muscle Enzyme Elevation in Different Myopathies
| Muscle Disease | CK-MM | Aldolase |
|---|---|---|
| Duchenne Muscular Dystrophy (DMD) | ↑↑↑ | ↑ |
| Becker Muscular Dystrophy (BMD) | ↑ | ↑ |
| Polymyositis/Dermatomyositis | ↑↑ | ↑ |
| Rhabdomyolysis | ↑↑↑ | Normal |
| Crush Injury | ↑↑↑ | Normal |
| Glycogen Storage Diseases (McArdle’s Disease) | ↑ | ↑ |
| ALS, Myasthenia Gravis | Normal | Normal |
5. Clinical Interpretation of CK and Aldolase
| Enzyme Pattern | Possible Diagnosis |
|---|---|
| CK-MM ↑, Aldolase ↑ | Muscular Dystrophy (DMD, BMD) |
| CK-MM ↑↑↑, Aldolase Normal | Rhabdomyolysis |
| CK-MM ↑, Aldolase ↑↑ | Polymyositis, Dermatomyositis |
| CK Normal, Aldolase Normal | Neurogenic Disorders (ALS, Myasthenia Gravis) |
6. Clinical Scenarios & Diagnostic Approach
Case 1: Young Boy with Progressive Muscle Weakness
- Symptoms: Difficulty walking, calf enlargement (pseudohypertrophy)
- Lab Findings: CK 10,000 U/L, Aldolase ↑, Myoglobin ↑
- Diagnosis: Duchenne Muscular Dystrophy (DMD)
Case 2: Adult with Sudden Muscle Pain After Exercise
- Symptoms: Severe leg pain, dark urine
- Lab Findings: CK > 10,000 U/L, Aldolase Normal, Myoglobin ↑↑
- Diagnosis: Rhabdomyolysis (Exercise-Induced)
Case 3: Middle-Aged Woman with Proximal Muscle Weakness
- Symptoms: Difficulty raising arms, muscle pain, skin rash
- Lab Findings: CK ↑↑, Aldolase ↑↑, LDH ↑
- Diagnosis: Polymyositis/Dermatomyositis.
Comprehensive Overview of Bone Diseases and Diagnostic Enzymes
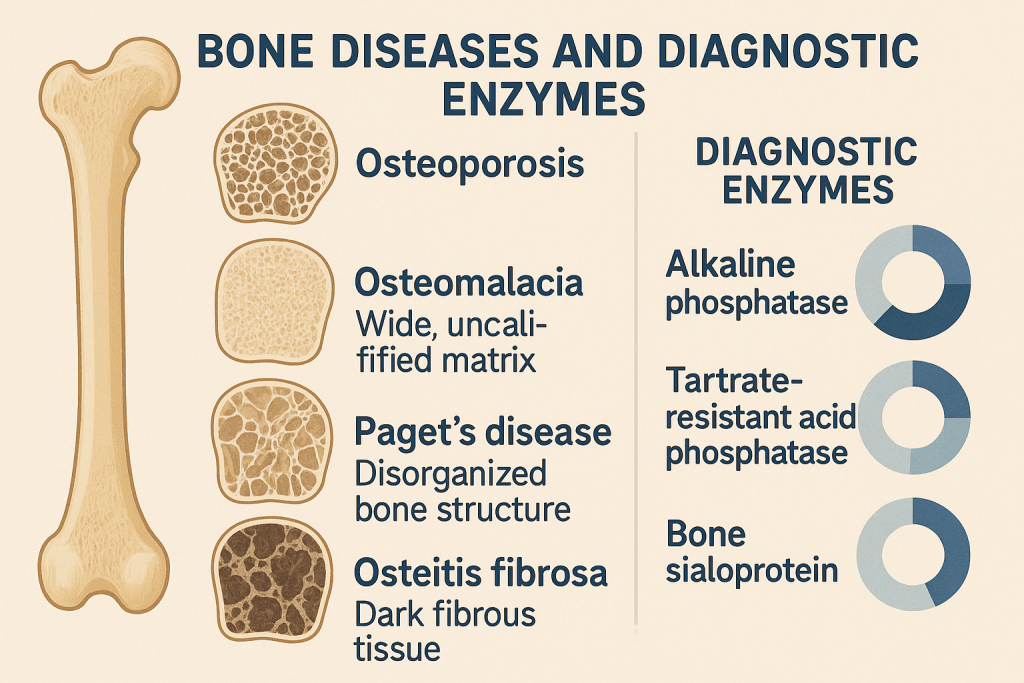
Introduction
Bone diseases encompass a variety of metabolic, degenerative, genetic, inflammatory, and neoplastic conditions that affect bone formation, resorption, and mineralization. Laboratory tests, particularly bone-related enzymes and biomarkers, play a crucial role in diagnosis, monitoring, and treatment of bone disorders.
This article provides a detailed analysis of key enzymes and biomarkers used in diagnosing bone diseases:
- Alkaline Phosphatase (ALP)
- Acid Phosphatase (ACP)
- Osteocalcin
- Tartrate-Resistant Acid Phosphatase (TRAP)
- Bone Resorption Markers (CTx, NTx, Hydroxyproline)
1. Alkaline Phosphatase (ALP) in Bone Diseases
Definition:
- ALP is an enzyme involved in bone mineralization and osteoblastic activity.
- It hydrolyzes phosphate esters, releasing phosphate ions for bone formation.
- Bone ALP is synthesized by osteoblasts (bone-forming cells).
Normal Range:
- Total ALP: 44-147 U/L
- Bone-Specific ALP (B-ALP): <20 U/L
Tissue Distribution:
- Bone (Osteoblasts)
- Liver (Bile Ducts)
- Intestines
- Placenta
Clinical Significance in Bone Diseases:
- ALP is a key marker of bone formation.
- Markedly increased in:
- Paget’s Disease of Bone (Osteitis Deformans)
- Rickets and Osteomalacia (Vitamin D Deficiency)
- Primary Hyperparathyroidism
- Osteoblastic Bone Tumors (Osteosarcoma, Bone Metastases)
- Mildly elevated in:
- Osteoporosis
- Fracture Healing
Differentiating Bone vs. Liver ALP:
- ALP is elevated in both bone and liver diseases.
- GGT helps differentiate:
- ALP ↑ & GGT ↑ → Liver disease
- ALP ↑ & GGT Normal → Bone disease
Bone ALP is a direct indicator of osteoblastic activity and bone formation.
2. Acid Phosphatase (ACP) in Bone Diseases
Definition:
- ACP is an enzyme involved in bone resorption and osteoclast activity.
- Prostatic ACP is a tumor marker for prostate cancer.
Normal Range:
- 0.5-5 U/L
Tissue Distribution:
- Bone (Osteoclasts)
- Prostate
- Liver, RBCs, Spleen
Clinical Significance in Bone Diseases:
- ACP is a marker of osteoclastic bone resorption.
- Elevated in:
- Paget’s Disease of Bone
- Osteolytic Bone Metastases
- Hyperparathyroidism
- Prostate Cancer with Bone Metastases
Prostate ACP is used to detect and monitor prostate cancer spread to bones.
3. Osteocalcin (Bone GLA Protein)
Definition:
- Osteocalcin is a protein secreted by osteoblasts during bone formation.
- It binds to calcium and hydroxyapatite, promoting mineralization.
Normal Range:
- Men: 3-13 ng/mL
- Women: 2-10 ng/mL
Clinical Significance in Bone Diseases:
- Osteocalcin is a direct marker of osteoblastic activity.
- Increased in:
- Osteoporosis
- Paget’s Disease of Bone
- Primary Hyperparathyroidism
- Decreased in:
- Osteomalacia
- Hypoparathyroidism
Osteocalcin is used to monitor osteoporosis and response to treatment.
4. Tartrate-Resistant Acid Phosphatase (TRAP)
Definition:
- TRAP is an enzyme secreted by osteoclasts, involved in bone resorption.
- It is used as a marker of osteoclastic activity.
Normal Range:
- <5 U/L
Clinical Significance in Bone Diseases:
- Elevated in:
- Paget’s Disease of Bone
- Osteolytic Bone Tumors
- Hyperparathyroidism
- Multiple Myeloma
- Osteoclastoma (Giant Cell Tumor of Bone)
TRAP is a specific marker for bone resorption and osteoclastic activity.
5. Bone Resorption Markers
A. C-Telopeptide (CTx) and N-Telopeptide (NTx)
- CTx and NTx are fragments of collagen released during bone breakdown.
- They are used to assess bone turnover in osteoporosis.
- Elevated in:
- Osteoporosis
- Hyperparathyroidism
- Paget’s Disease
B. Hydroxyproline
- Amino acid released from collagen degradation.
- Elevated in:
- Paget’s Disease
- Osteoporosis
- Bone Tumors
6. Enzyme Elevation in Different Bone Diseases
| Bone Disease | ALP | ACP | Osteocalcin | TRAP | CTx/NTx |
|---|---|---|---|---|---|
| Osteoporosis | Normal/Slight ↑ | Normal | ↑ | Normal/↑ | ↑ |
| Paget’s Disease | ↑↑↑ | ↑ | ↑ | ↑↑ | ↑↑ |
| Rickets/Osteomalacia | ↑ | Normal | ↓ | Normal | ↑ |
| Hyperparathyroidism | ↑ | ↑ | ↑ | ↑ | ↑ |
| Osteolytic Bone Tumors | ↑ | ↑ | Normal | ↑ | ↑ |
| Osteosarcoma (Bone Cancer) | ↑ | Normal | ↑ | Normal | Normal |
7. Clinical Interpretation of Bone Enzymes
| Enzyme Pattern | Possible Diagnosis |
|---|---|
| ALP ↑, GGT Normal | Bone Disease |
| ALP ↑, GGT ↑ | Liver Disease |
| ALP ↑↑, ACP ↑ | Paget’s Disease, Bone Tumors |
| Osteocalcin ↑, CTx/NTx ↑ | Osteoporosis |
| ALP ↑, Osteocalcin ↓ | Osteomalacia |
8. Clinical Cases & Diagnostic Approach
Case 1: Elderly Woman with Hip Fracture
- Symptoms: Chronic back pain, height loss
- Lab Findings: ALP Normal, Osteocalcin ↑, CTx ↑
- Diagnosis: Osteoporosis
Case 2: Middle-Aged Man with Bone Pain & Skull Enlargement
- Symptoms: Bone pain, deformities
- Lab Findings: ALP ↑↑, ACP ↑, TRAP ↑
- Diagnosis: Paget’s Disease of Bone
Case 3: Young Child with Bowed Legs
- Symptoms: Delayed growth, muscle weakness
- Lab Findings: ALP ↑, Osteocalcin ↓
- Diagnosis: Rickets (Vitamin D Deficiency)
Comprehensive Overview of Alkaline Phosphatase (ALP) in Bone Diseases
Introduction
Alkaline Phosphatase (ALP) is an enzyme essential for bone mineralization and metabolism. It is widely used as a biomarker for bone diseases, liver diseases, and certain metabolic conditions.
This article focuses on ALP’s role in bone health, including its normal range, tissue distribution, clinical significance, and interpretation in different bone disorders.
1. Alkaline Phosphatase (ALP) – Definition & Function
- ALP is a hydrolase enzyme that removes phosphate groups from molecules.
- Bone-specific ALP (B-ALP) is produced by osteoblasts and plays a key role in bone mineralization.
- It is a marker of bone formation and is elevated in conditions that increase osteoblastic activity.
2. Normal Range of ALP
| Group | Normal ALP Level (U/L) |
|---|---|
| Adults | 44-147 U/L |
| Children & Adolescents | 100-500 U/L (Higher due to bone growth) |
| Pregnant Women | Elevated (Placental ALP) |
In children and teenagers, ALP is physiologically high due to active bone growth.
3. Tissue Distribution of ALP
| Tissue | ALP Isoenzyme |
|---|---|
| Bone (Osteoblasts) | Bone-Specific ALP (B-ALP) |
| Liver (Biliary Ducts) | Liver ALP |
| Placenta | Placental ALP |
| Intestines | Intestinal ALP |
| Kidney | Renal ALP |
Bone and liver are the major sources of ALP in blood.
4. Clinical Significance of ALP in Bone Diseases
- ALP is a primary marker for bone formation.
- It is elevated in conditions with increased osteoblastic activity.
- Bone-Specific ALP (B-ALP) is useful for differentiating bone vs. liver ALP elevation.
Conditions with Increased ALP (Bone Diseases)
| Bone Disease | ALP Level | Pathophysiology |
|---|---|---|
| Paget’s Disease of Bone | ↑↑↑ | Excessive bone turnover with abnormal remodeling |
| Rickets & Osteomalacia | ↑ | Defective mineralization due to Vitamin D deficiency |
| Osteoblastic Bone Tumors (Osteosarcoma, Bone Metastases) | ↑ | Increased osteoblast activity |
| Primary Hyperparathyroidism | ↑ | Increased bone resorption leading to compensatory bone formation |
| Fracture Healing | ↑ | Bone repair increases osteoblastic activity |
Conditions with Normal or Slightly Elevated ALP
| Condition | ALP Level | Comment |
|---|---|---|
| Osteoporosis | Normal/Slight ↑ | ALP is not significantly elevated unless there is a fracture |
| Chronic Kidney Disease (CKD-MBD) | ↑ | Secondary hyperparathyroidism increases bone turnover |
| Pregnancy | ↑ | Placental ALP increases |
5. Differentiating Bone vs. Liver ALP
- ALP is elevated in both bone and liver diseases.
- How to distinguish the source?
- Bone Disease: ALP ↑, GGT Normal
- Liver Disease: ALP ↑, GGT ↑
- Bone-Specific ALP (B-ALP) and serum calcium help confirm bone involvement.
6. ALP in Different Bone Diseases
| Bone Disease | ALP Level | Other Markers |
|---|---|---|
| Paget’s Disease | ↑↑↑ | Osteocalcin ↑, Hydroxyproline ↑ |
| Rickets/Osteomalacia | ↑ | Calcium ↓, Vitamin D ↓, PTH ↑ |
| Hyperparathyroidism | ↑ | Calcium ↑, PTH ↑ |
| Osteosarcoma (Bone Cancer) | ↑ | LDH ↑, Tumor Markers ↑ |
| Fracture Healing | ↑ | Calcium Normal, CTx/NTx ↑ |
Paget’s Disease of Bone shows the highest ALP elevation in bone disorders.
7. ALP in Paget’s Disease of Bone
Paget’s Disease – Overview
- Characterized by excessive bone remodeling
- Increased osteoclastic and osteoblastic activity
- ALP is a key diagnostic marker
ALP in Paget’s Disease
| Stage | ALP Level |
|---|---|
| Active Disease | ↑↑↑ (3-10x normal) |
| Treatment with Bisphosphonates | Decreases ALP |
ALP is used to monitor treatment response in Paget’s Disease.
8. ALP in Rickets & Osteomalacia
Definition
- Rickets (Children) & Osteomalacia (Adults) are caused by Vitamin D deficiency leading to defective bone mineralization.
ALP in Rickets/Osteomalacia
- Elevated ALP due to compensatory osteoblastic activity.
- Other findings:
- Low calcium
- Low phosphate
- High parathyroid hormone (PTH)
Vitamin D supplementation normalizes ALP levels over time.
9. ALP in Osteoporosis
- Osteoporosis primarily affects bone density, not turnover.
- ALP is usually normal.
- Elevated ALP may be seen in:
- Fractures due to osteoporosis
- High-turnover osteoporosis (seen in hyperparathyroidism or CKD-MBD)
For osteoporosis monitoring, Bone-Specific ALP, Osteocalcin, and CTx/NTx are more useful than total ALP.
10. Interpretation of ALP in Bone and Liver Disorders
| ALP Pattern | Possible Diagnosis |
|---|---|
| ALP ↑, GGT Normal | Bone Disease |
| ALP ↑, GGT ↑ | Liver Disease |
| ALP ↑↑↑ (>500 U/L) | Paget’s Disease, Osteosarcoma |
| ALP ↑, Calcium ↓ | Rickets, Osteomalacia |
| ALP ↑, PTH ↑ | Hyperparathyroidism |
11. Clinical Cases & Diagnostic Approach
Case 1: Elderly Man with Bone Pain & Deformities
- Symptoms: Bone pain, skull thickening
- Lab Findings: ALP ↑↑↑ (500 U/L), GGT Normal, Osteocalcin ↑
- Diagnosis: Paget’s Disease of Bone
Case 2: Child with Bowed Legs & Weakness
- Symptoms: Growth delay, bone deformities
- Lab Findings: ALP ↑, Calcium ↓, Vitamin D ↓
- Diagnosis: Rickets (Vitamin D Deficiency)
Case 3: Postmenopausal Woman with Fractures
- Symptoms: Chronic back pain, height loss
- Lab Findings: ALP Normal, CTx/NTx ↑, Osteocalcin ↑
- Diagnosis: Osteoporosis.
Comprehensive Overview of Prostate Cancer and Diagnostic Enzymes
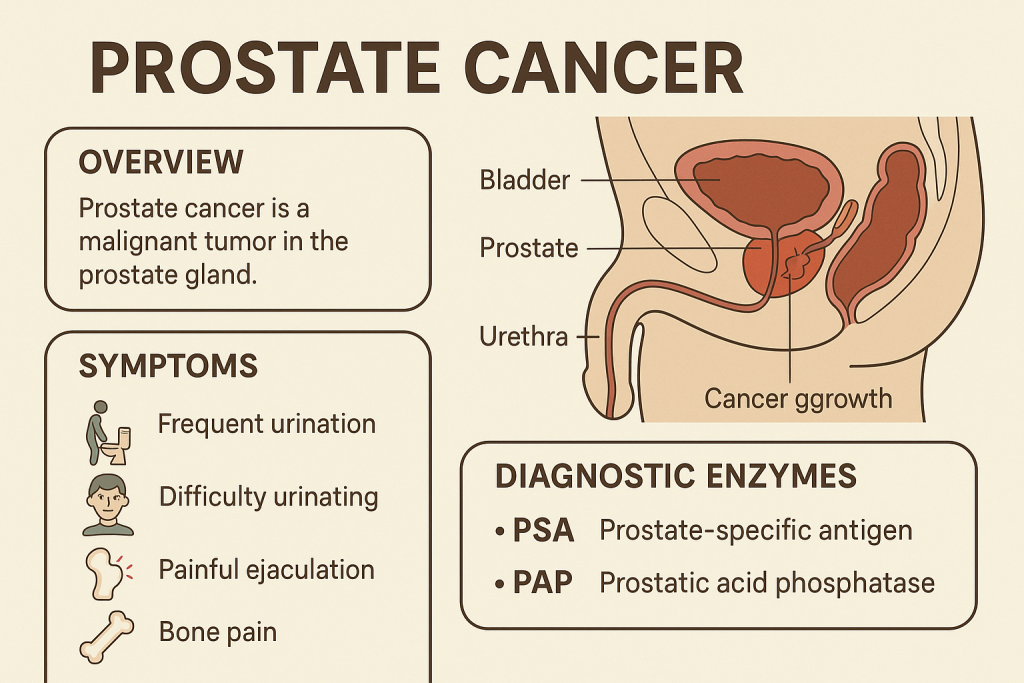
Introduction
Prostate cancer is one of the most common cancers in men, affecting the prostate gland, which produces seminal fluid. Early diagnosis is crucial for effective treatment. Biochemical markers and enzymes, including Acid Phosphatase (ACP) and Prostate-Specific Antigen (PSA), play a key role in diagnosing, staging, and monitoring prostate cancer.
This article provides a detailed analysis of enzymatic markers in prostate cancer, including Acid Phosphatase (ACP), Prostate-Specific Antigen (PSA), and Alkaline Phosphatase (ALP).
1. Key Enzymatic Markers in Prostate Cancer
| Marker | Function | Clinical Significance |
|---|---|---|
| Acid Phosphatase (ACP) | Hydrolyzes phosphate esters | Elevated in prostate cancer, especially with bone metastases |
| Prostate-Specific Antigen (PSA) | Liquefies semen | Primary marker for prostate cancer diagnosis and monitoring |
| Alkaline Phosphatase (ALP) | Bone mineralization | Elevated in prostate cancer with bone metastases |
2. Acid Phosphatase (ACP) in Prostate Cancer
Definition:
- Acid Phosphatase (ACP) is an enzyme that hydrolyzes phosphate esters at an acidic pH.
- Prostatic Acid Phosphatase (PAP) is a subtype of ACP produced in the prostate gland.
Normal Range:
- Total ACP: 0.5 – 5.5 U/L
- Prostatic ACP (PAP): <3 ng/mL
Tissue Distribution:
| ACP Isoenzyme | Primary Location |
|---|---|
| Prostatic ACP (PAP) | Prostate gland |
| Lysosomal ACP | Liver, Kidney, RBCs |
| Bone ACP | Osteoclasts |
Clinical Significance in Prostate Cancer:
- Elevated ACP is associated with advanced prostate cancer and metastasis.
- Markedly increased in:
- Metastatic Prostate Cancer (Bone Involvement)
- Locally Advanced Prostate Tumors
- Prostate Cancer Recurrence
- Slightly increased in:
- Benign Prostatic Hyperplasia (BPH)
- Prostatitis (Inflammation of the prostate)
Prostatic ACP is useful for staging and monitoring prostate cancer but is less commonly used due to PSA’s higher sensitivity.
3. Prostate-Specific Antigen (PSA)
Definition:
- PSA is a glycoprotein enzyme secreted by prostate epithelial cells.
- It helps liquefy semen and breaks down seminal coagulum.
Normal Range:
| Age Group | Normal PSA Level (ng/mL) |
|---|---|
| 40-49 years | 0-2.5 |
| 50-59 years | 0-3.5 |
| 60-69 years | 0-4.5 |
| >70 years | 0-6.5 |
Clinical Significance in Prostate Cancer:
- PSA is the most sensitive marker for prostate cancer screening.
- Elevated PSA in:
- Prostate Cancer (>10 ng/mL is highly suspicious)
- Benign Prostatic Hyperplasia (BPH)
- Prostatitis (Infections, Inflammation)
- PSA > 4 ng/mL requires further investigation (Biopsy recommended if PSA >10 ng/mL).
PSA in Prostate Cancer Staging:
| PSA Level (ng/mL) | Possible Diagnosis |
|---|---|
| <4 | Normal or BPH |
| 4-10 | Suspicious, may require biopsy |
| >10 | High suspicion for prostate cancer |
| >20 | Likely metastasis, further imaging required |
PSA is the primary biomarker for prostate cancer screening, treatment monitoring, and recurrence detection.
4. Alkaline Phosphatase (ALP) in Prostate Cancer
Definition:
- ALP is an enzyme involved in bone mineralization and is elevated in bone metastases.
- Prostate cancer often spreads to bones (osteoblastic metastases), causing an increase in ALP.
Normal Range:
- 44-147 U/L
Clinical Significance in Prostate Cancer:
- ALP is a marker of bone metastases in advanced prostate cancer.
- Elevated in:
- Prostate Cancer with Bone Metastases
- Paget’s Disease of Bone
- Osteoblastic Bone Tumors
High ALP in prostate cancer suggests bone metastases, requiring imaging (Bone Scan, MRI).
5. Biomarker Elevation in Different Prostate Conditions
| Condition | PSA | ACP (PAP) | ALP |
|---|---|---|---|
| Localized Prostate Cancer | ↑ | ↑ | Normal |
| Metastatic Prostate Cancer (Bone Metastases) | ↑↑↑ | ↑↑ | ↑↑ |
| Benign Prostatic Hyperplasia (BPH) | ↑ | Normal/Slight ↑ | Normal |
| Prostatitis | ↑ | Normal | Normal |
PSA is the best screening tool, while ACP and ALP help in staging and metastasis detection.
6. Clinical Interpretation of Prostate Enzymes
| Biomarker Pattern | Possible Diagnosis |
|---|---|
| PSA ↑, ACP Normal | Early Prostate Cancer, BPH |
| PSA ↑↑, ACP ↑ | Prostate Cancer |
| PSA ↑↑↑, ACP ↑↑, ALP ↑↑ | Prostate Cancer with Bone Metastases |
| ALP ↑, PSA Normal | Bone Disease (Paget’s, Osteosarcoma) |
7. Clinical Cases & Diagnostic Approach
Case 1: Elderly Male with Urinary Symptoms
- Symptoms: Frequent urination, weak urine stream
- Lab Findings: PSA 6 ng/mL, ACP Normal, ALP Normal
- Diagnosis: Benign Prostatic Hyperplasia (BPH)
Case 2: 65-Year-Old Male with Back Pain & Fatigue
- Symptoms: Chronic bone pain, fatigue
- Lab Findings: PSA 30 ng/mL, ACP ↑, ALP ↑↑
- Diagnosis: Prostate Cancer with Bone Metastases
Case 3: 55-Year-Old Male with Elevated PSA
- Symptoms: No symptoms, PSA found elevated on routine check
- Lab Findings: PSA 12 ng/mL, ACP Slight ↑, ALP Normal
- Next Step: Prostate Biopsy for Cancer Confirmation.
Comprehensive Overview of Prostate-Specific Antigen (PSA) and Acid Phosphatase (ACP) in Prostate Cancer
Introduction
Prostate cancer is one of the most common malignancies in men. Prostate-Specific Antigen (PSA) and Acid Phosphatase (ACP) are key biomarkers for screening, diagnosis, staging, and monitoring of prostate cancer.
This article provides detailed insights into the clinical significance, normal ranges, and interpretation of PSA and ACP in prostate cancer.
1. Prostate-Specific Antigen (PSA)
Definition:
- PSA is a glycoprotein enzyme produced by the prostate epithelial cells.
- It functions to liquefy semen, aiding sperm motility.
- Elevated PSA levels indicate prostate pathology, including cancer, BPH, or prostatitis.
Normal Range:
| Age Group | Normal PSA Level (ng/mL) |
|---|---|
| 40-49 years | 0-2.5 |
| 50-59 years | 0-3.5 |
| 60-69 years | 0-4.5 |
| >70 years | 0-6.5 |
PSA levels naturally increase with age.
Clinical Significance in Prostate Cancer:
- PSA is the most sensitive biomarker for prostate cancer screening.
- Elevated PSA in:
- Prostate Cancer (>10 ng/mL is highly suspicious)
- Benign Prostatic Hyperplasia (BPH)
- Prostatitis (Infections, Inflammation of the prostate)
PSA Levels and Cancer Risk:
| PSA Level (ng/mL) | Possible Diagnosis | Next Step |
|---|---|---|
| <4 | Normal or BPH | Routine follow-up |
| 4-10 | Suspicious for cancer | Consider biopsy |
| >10 | High suspicion of prostate cancer | Prostate biopsy |
| >20 | Likely metastatic prostate cancer | Imaging (Bone Scan, MRI) |
PSA > 10 ng/mL is a strong indicator of prostate cancer and requires further testing.
PSA Velocity & PSA Doubling Time:
- PSA Velocity: Rate of PSA increase over time (>0.75 ng/mL per year is suspicious for cancer).
- PSA Doubling Time: The time it takes for PSA levels to double (Shorter doubling time suggests aggressive prostate cancer).
PSA Density (PSAD):
- PSAD = PSA level (ng/mL) / Prostate volume (mL)
- PSAD > 0.15 suggests higher likelihood of prostate cancer.
PSA in Monitoring Prostate Cancer:
- Post-Treatment PSA Levels:
- After Prostatectomy: PSA should be undetectable (<0.01 ng/mL).
- After Radiation Therapy: PSA should gradually decrease (PSA <2 ng/mL is a good response).
- PSA Recurrence: Rise in PSA after treatment suggests recurrence or metastasis.
2. Acid Phosphatase (ACP)
Definition:
- Acid Phosphatase (ACP) is an enzyme that hydrolyzes phosphate esters at an acidic pH.
- Prostatic Acid Phosphatase (PAP) is a subtype of ACP produced by the prostate.
- Historically used for prostate cancer diagnosis, but now replaced by PSA.
Normal Range:
- Total ACP: 0.5 – 5.5 U/L
- Prostatic ACP (PAP): <3 ng/mL
Tissue Distribution:
| ACP Isoenzyme | Primary Location |
|---|---|
| Prostatic ACP (PAP) | Prostate Gland |
| Lysosomal ACP | Liver, Kidney, RBCs |
| Bone ACP | Osteoclasts |
Clinical Significance in Prostate Cancer:
- Elevated ACP is associated with advanced prostate cancer and metastasis.
- Markedly increased in:
- Metastatic Prostate Cancer (Bone Involvement)
- Locally Advanced Prostate Tumors
- Prostate Cancer Recurrence
- Slightly increased in:
- Benign Prostatic Hyperplasia (BPH)
- Prostatitis (Inflammation of the prostate)
Prostatic ACP (PAP) is less sensitive than PSA but useful in detecting advanced prostate cancer and bone metastases.
3. PSA vs. ACP in Prostate Cancer
| Feature | Prostate-Specific Antigen (PSA) | Acid Phosphatase (ACP/PAP) |
|---|---|---|
| Primary Function | Semen liquefaction | Phosphate metabolism |
| Most Specific For | Prostate Cancer (Primary Marker) | Prostate Cancer with Bone Metastases |
| Normal Range | 0-4 ng/mL (Varies with age) | 0.5-5.5 U/L |
| Elevated in | Prostate Cancer, BPH, Prostatitis | Metastatic Prostate Cancer |
| Best Used For | Screening, Early Diagnosis, Monitoring | Staging, Metastasis Detection |
| Main Disadvantage | False positives in BPH & prostatitis | Less sensitive than PSA |
PSA is the preferred screening test, while ACP is useful in detecting metastases.
4. Biomarker Elevation in Different Prostate Conditions
| Condition | PSA | ACP (PAP) |
|---|---|---|
| Localized Prostate Cancer | ↑ | Normal/Slight ↑ |
| Metastatic Prostate Cancer (Bone Metastases) | ↑↑↑ | ↑↑ |
| Benign Prostatic Hyperplasia (BPH) | ↑ | Normal |
| Prostatitis | ↑ | Normal |
PSA is the best screening tool, while ACP helps assess metastasis.
5. Clinical Interpretation of PSA and ACP
| Biomarker Pattern | Possible Diagnosis |
|---|---|
| PSA ↑, ACP Normal | Early Prostate Cancer, BPH |
| PSA ↑↑, ACP ↑ | Prostate Cancer |
| PSA ↑↑↑, ACP ↑↑ | Prostate Cancer with Bone Metastases |
6. Clinical Cases & Diagnostic Approach
Case 1: 60-Year-Old Man with Urinary Symptoms
- Symptoms: Frequent urination, weak urine stream
- Lab Findings: PSA 6 ng/mL, ACP Normal
- Diagnosis: Benign Prostatic Hyperplasia (BPH)
Case 2: 70-Year-Old Man with Back Pain & Fatigue
- Symptoms: Chronic bone pain, weight loss
- Lab Findings: PSA 30 ng/mL, ACP ↑
- Diagnosis: Prostate Cancer with Bone Metastases
Case 3: 55-Year-Old Man with Elevated PSA on Screening
- Symptoms: No symptoms, routine PSA check
- Lab Findings: PSA 12 ng/mL, ACP Slight ↑
- Next Step: Prostate Biopsy for Cancer Confirmation.