BSC SEM 1 UNIT 3 APPLIED NUTRITION AND DIETETICS
UNIT 3 PROTEIN

Introduction to Protein.
Proteins are essential macronutrients composed of amino acids, which play a crucial role in growth, repair, and maintenance of body tissues. They are often referred to as the “building blocks of life” because they are involved in almost every biological process in the human body.
Definition of Protein
Proteins are complex organic molecules made up of carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and sometimes sulfur (S). They are polymers of amino acids linked together by peptide bonds.
Importance of Protein in Nutrition
- Growth and Development – Proteins are essential for the growth of body tissues, especially during infancy, childhood, adolescence, and pregnancy.
- Tissue Repair and Maintenance – Helps in healing wounds and repairing damaged tissues.
- Enzyme and Hormone Production – Many enzymes and hormones (such as insulin and hemoglobin) are proteins that regulate physiological functions.
- Immune System Support – Antibodies that fight infections are proteins.
- Energy Source – In the absence of carbohydrates and fats, proteins can be used for energy production.
- Muscle Contraction and Movement – Proteins like actin and myosin help in muscle contractions.
- Transportation and Storage – Proteins like hemoglobin transport oxygen, and albumin helps in nutrient distribution.
Sources of Protein

1. Animal Sources (Complete Proteins)
- Meat (chicken, beef, pork)
- Fish and seafood
- Eggs
- Dairy products (milk, cheese, yogurt)
2. Plant Sources (Incomplete Proteins)
- Legumes (beans, lentils, chickpeas)
- Nuts and seeds (almonds, peanuts, flaxseeds)
- Whole grains (quinoa, oats, brown rice)
- Soy products (tofu, soy milk)
Types of Proteins
- Complete Proteins – Contain all essential amino acids (e.g., animal proteins, soy, quinoa).
- Incomplete Proteins – Lack one or more essential amino acids (e.g., most plant-based proteins).
- Complementary Proteins – A combination of two incomplete proteins to provide all essential amino acids (e.g., rice and beans).
Recommended Daily Allowance (RDA)
- Infants: 1.5 g/kg body weight/day
- Children: 1.0 – 1.2 g/kg body weight/day
- Adults: 0.8 g/kg body weight/day
- Pregnant and Lactating Women: 1.1 – 1.3 g/kg body weight/day
- Athletes and Bodybuilders: 1.2 – 2.0 g/kg body weight/day
Deficiency of Protein (Protein-Energy Malnutrition – PEM)
- Kwashiorkor – Characterized by edema, muscle wasting, skin changes, and irritability.
- Marasmus – Severe wasting, stunted growth, and extreme thinness due to prolonged protein and calorie deficiency.
Excess Protein Intake and Its Effects
- Kidney Strain – High protein intake can increase kidney workload.
- Dehydration – Excess protein metabolism requires more water.
- Increased Risk of Osteoporosis – High protein intake may lead to calcium loss.
- Weight Gain – Excess protein is converted into fat if not utilized.
Composition of Protein
Introduction
Proteins are vital macronutrients essential for growth, repair, and various physiological functions in the human body. They are made up of amino acids, which are the fundamental building blocks of life. Understanding the composition of proteins is crucial for nursing and healthcare professionals to provide optimal nutritional care to patients.
1. Composition of Proteins
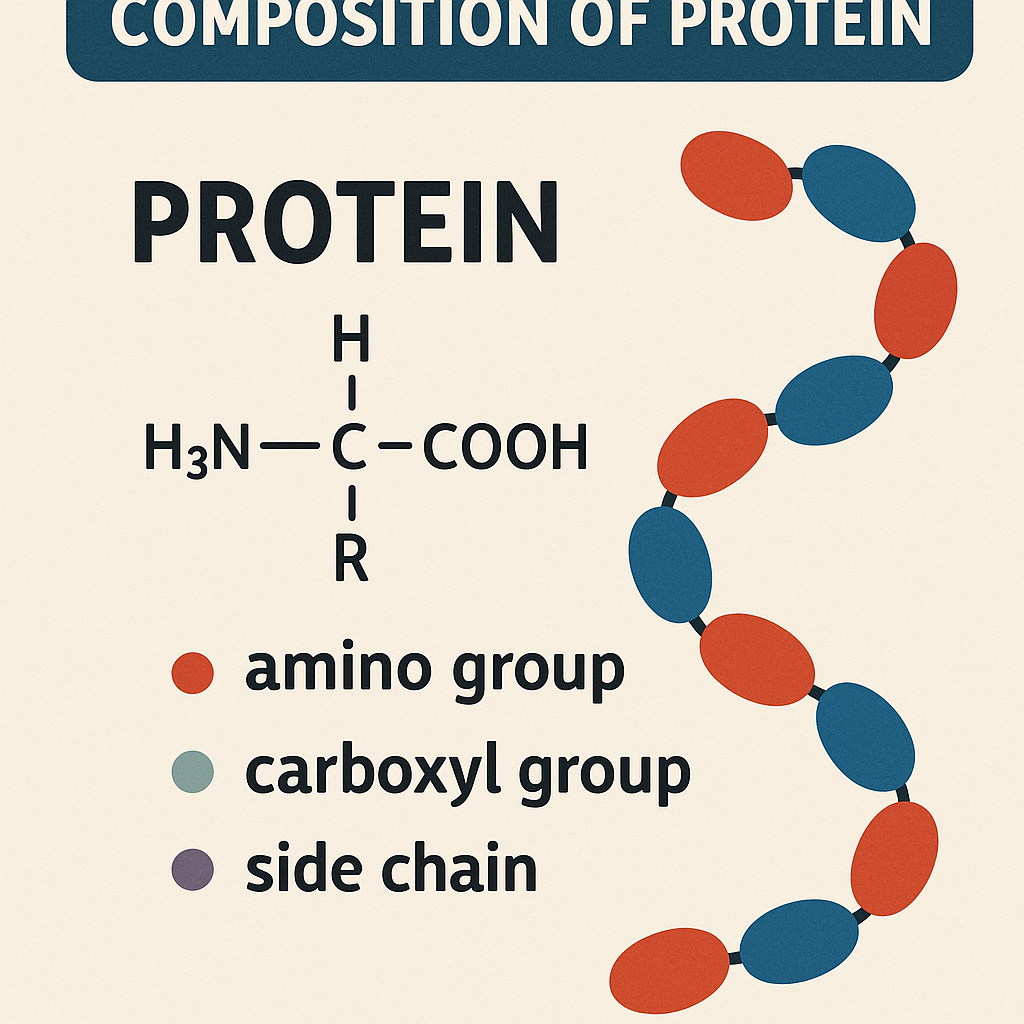
Proteins are composed of the following elements:
- Carbon (C) – 50–55%
- Hydrogen (H) – 6–7%
- Oxygen (O) – 20–23%
- Nitrogen (N) – 15–18% (This is the distinguishing element of proteins)
- Sulfur (S) – 0.5–2% (Present in some amino acids like cysteine and methionine)
- Phosphorus (P) – Trace amounts (Found in some specialized proteins like casein)
These elements combine to form amino acids, which link together via peptide bonds to form proteins.
2. Amino Acids: The Building Blocks of Proteins
Proteins are made up of 20 different amino acids, which are classified into three categories:
A. Essential Amino Acids (Indispensable Amino Acids)
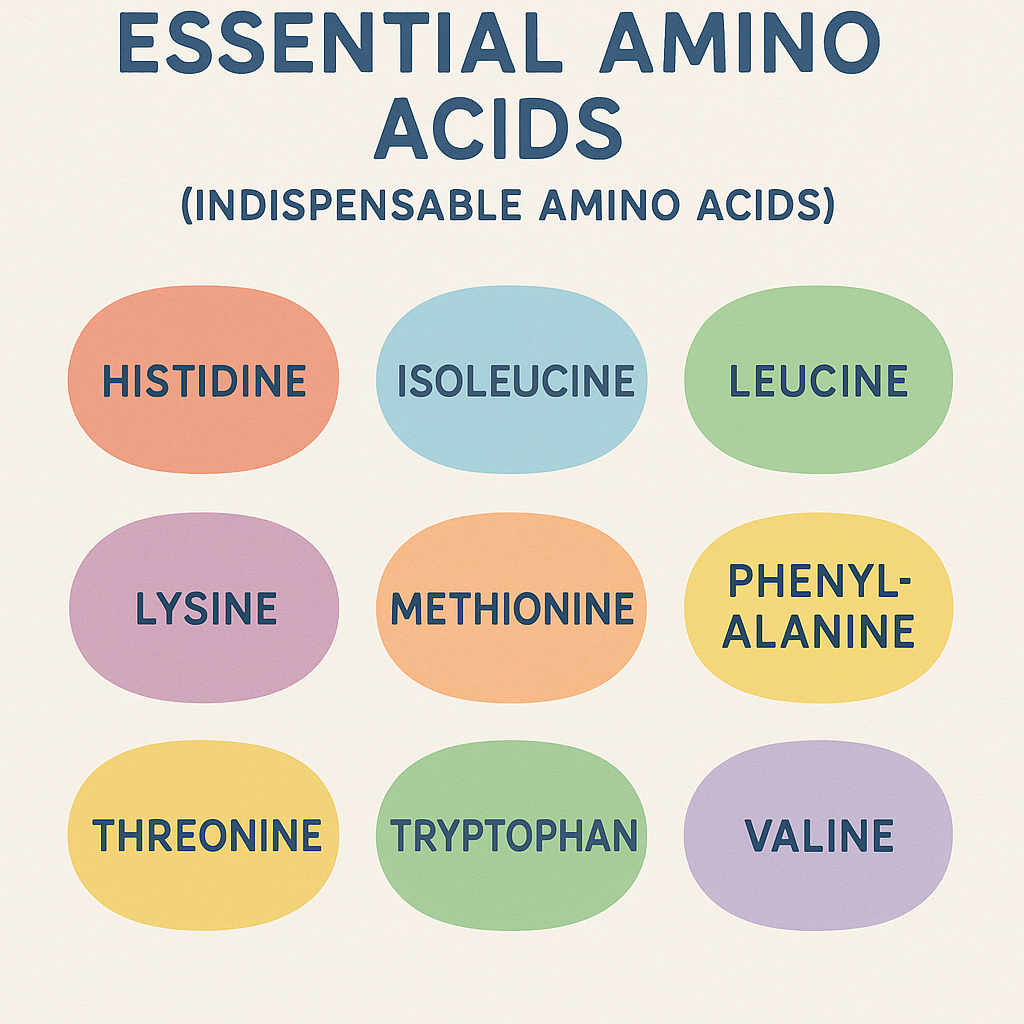
- Definition: These amino acids cannot be synthesized by the human body and must be obtained from the diet.
- List of Essential Amino Acids (Mnemonic: PVT TIM HALL)
- Phenylalanine
- Valine
- Tryptophan
- Threonine
- Isoleucine
- Methionine
- Histidine (Essential for infants)
- Arginine (Essential for infants)
- Leucine
- Lysine
B. Non-Essential Amino Acids (Dispensable Amino Acids)
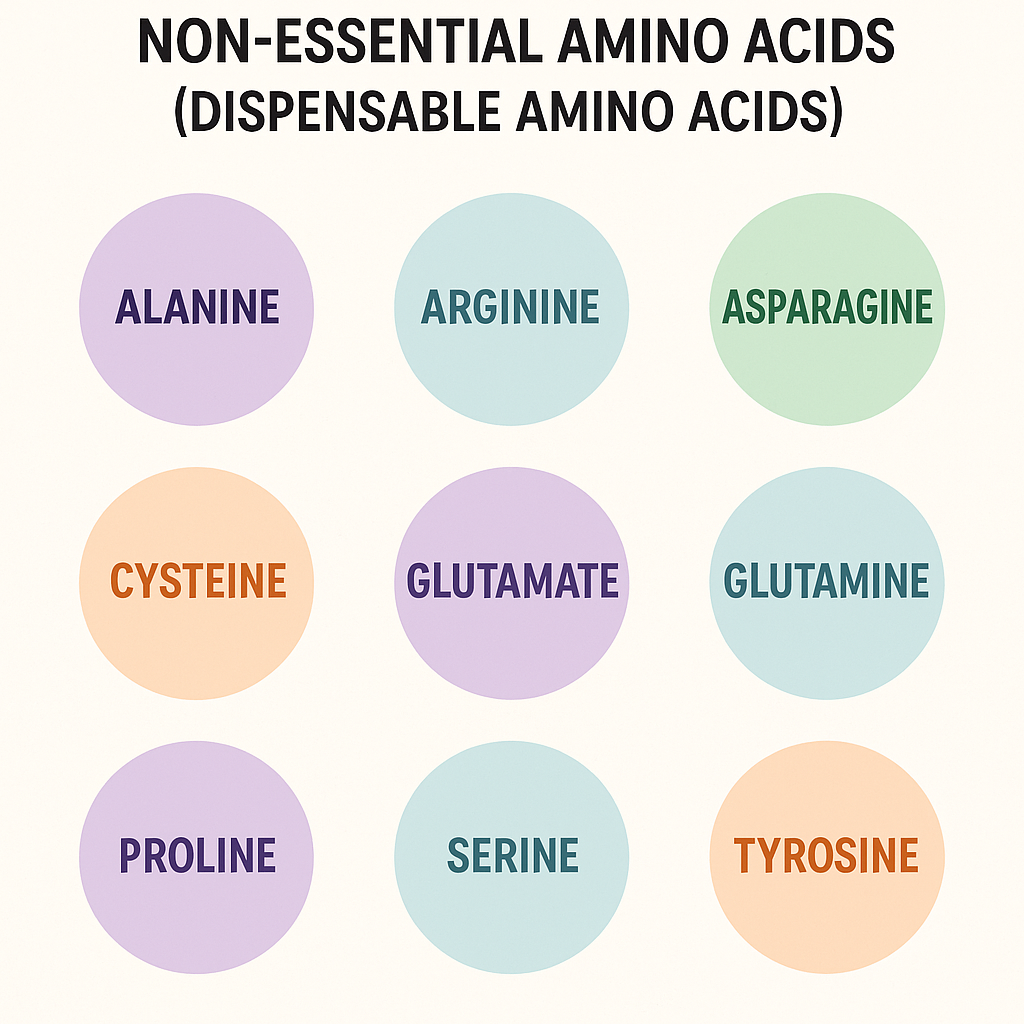
- Definition: These amino acids can be synthesized by the body.
- Examples:
- Alanine
- Aspartic acid
- Asparagine
- Glutamic acid
- Serine
C. Conditionally Essential Amino Acids
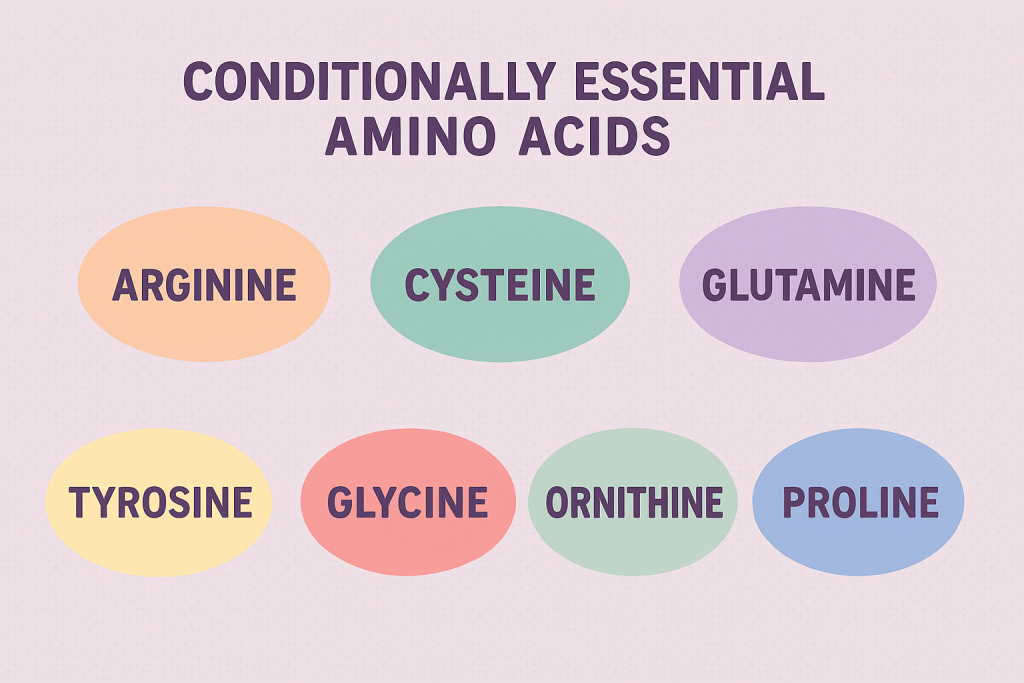
- Definition: These amino acids are usually non-essential but become essential in certain conditions, such as illness, stress, or metabolic disorders.
- Examples:
- Arginine
- Cysteine
- Glutamine
- Tyrosine
- Proline
- Glycine
3. Protein Structure
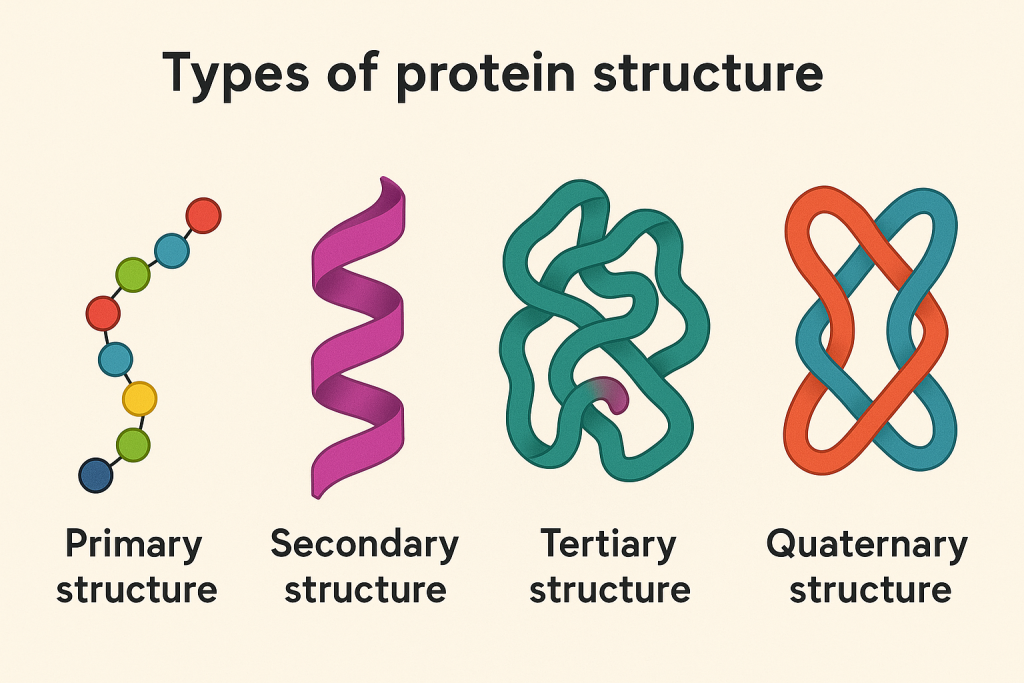
Proteins have four levels of structure that determine their function:
A. Primary Structure
- The linear sequence of amino acids in a polypeptide chain.
B. Secondary Structure
- Folding of the polypeptide chain due to hydrogen bonding.
- Examples:
- Alpha-helix (e.g., keratin in hair and nails)
- Beta-pleated sheets (e.g., silk proteins)
C. Tertiary Structure
- Three-dimensional folding of the protein due to interactions between side chains (R-groups).
- Determines the functionality of the protein (e.g., enzymes, hormones).
D. Quaternary Structure
- Complex structure formed by multiple polypeptide chains.
- Example: Hemoglobin (made of four polypeptide chains).
4. Classification of Proteins Based on Composition
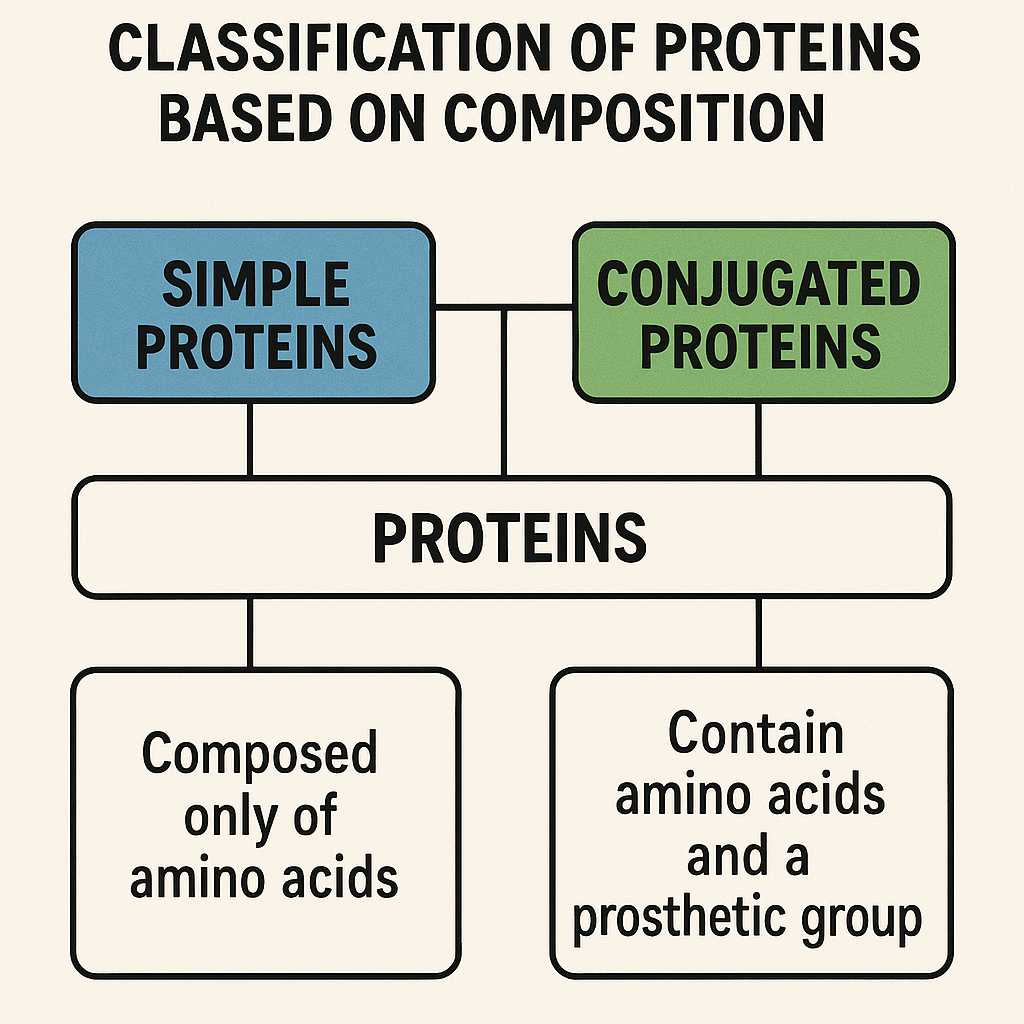
Proteins are classified into different types based on their chemical composition:
A. Simple Proteins
- Made up of only amino acids.
- Examples:
- Albumin (found in egg white, blood plasma)
- Globulin (found in blood and milk)
- Collagen (found in connective tissues)
B. Conjugated Proteins
- Contain a non-protein group (prosthetic group) along with amino acids.
- Examples:
- Glycoproteins – Protein + Carbohydrate (e.g., mucin)
- Lipoproteins – Protein + Lipid (e.g., HDL, LDL)
- Phosphoproteins – Protein + Phosphate (e.g., casein in milk)
- Hemoproteins – Protein + Iron (e.g., hemoglobin)
- Nucleoproteins – Protein + Nucleic Acid (e.g., ribosomes)
C. Derived Proteins
- Breakdown products of proteins.
- Examples:
- Peptones
- Proteoses
5. Classification of Proteins Based on Function
Proteins serve various physiological roles, classified into:
| Type of Protein | Function | Examples |
|---|---|---|
| Structural Proteins | Provide support | Collagen, Keratin |
| Enzymatic Proteins | Catalyze biochemical reactions | Amylase, Pepsin |
| Transport Proteins | Carry molecules | Hemoglobin, Albumin |
| Hormonal Proteins | Regulate metabolism | Insulin, Growth Hormone |
| Immune Proteins | Defense against infections | Antibodies (Immunoglobulins) |
| Contractile Proteins | Muscle contraction | Actin, Myosin |
| Storage Proteins | Store amino acids | Ferritin (iron storage), Casein (milk protein) |
6. Protein Metabolism
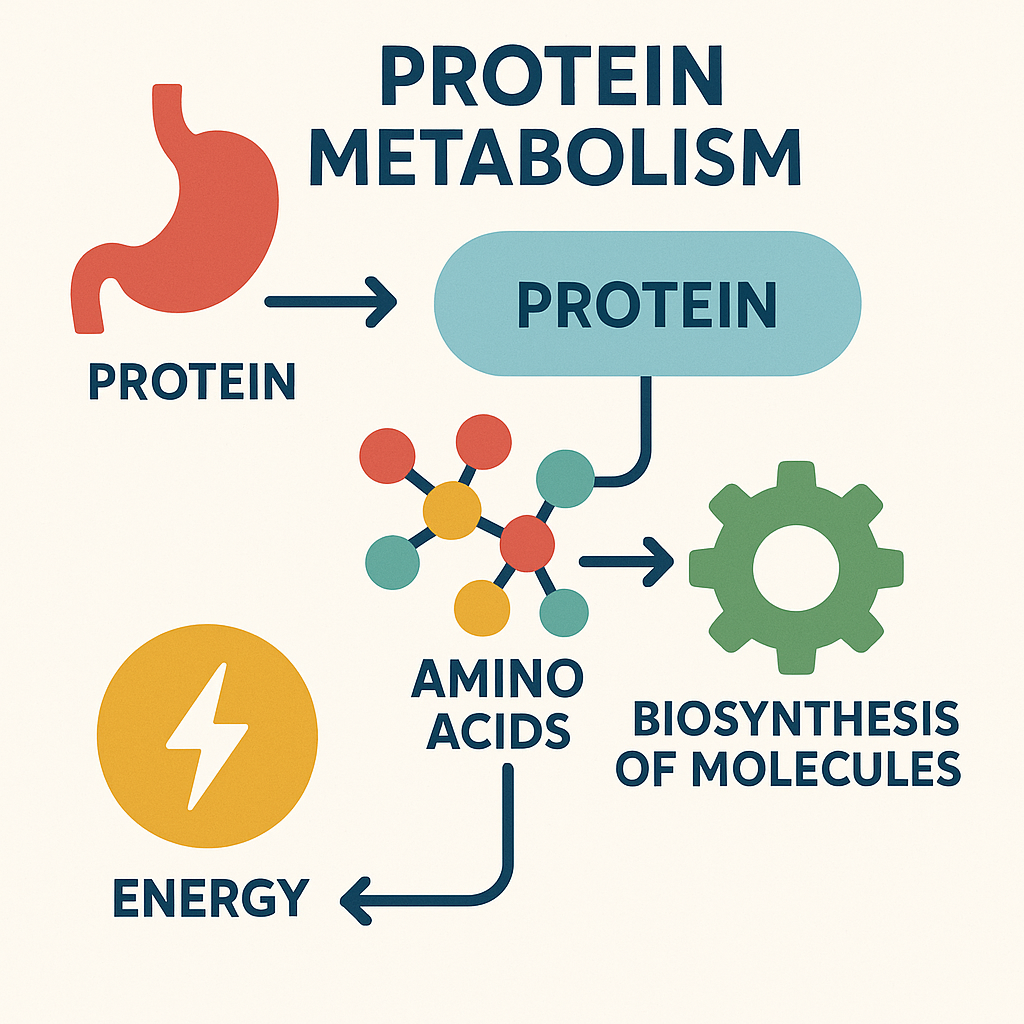
Protein metabolism involves digestion, absorption, and utilization of proteins.
A. Digestion of Proteins
- Mouth – No digestion of proteins.
- Stomach – Enzyme pepsin breaks down proteins into polypeptides.
- Small Intestine:
- Pancreatic enzymes: Trypsin, Chymotrypsin, Carboxypeptidase further break down polypeptides.
- Intestinal enzymes: Peptidases convert polypeptides into amino acids.
B. Absorption
- Amino acids are absorbed into the bloodstream through the small intestine and transported to the liver.
C. Utilization
- Proteins are used for:
- Tissue repair and growth
- Enzyme and hormone production
- Energy production (if carbohydrate and fat intake is insufficient)
D. Deamination and Excretion
- Excess amino acids undergo deamination in the liver, producing ammonia, which is converted into urea and excreted via the kidneys.
7. Importance of Protein in Nursing and Healthcare
- Wound Healing – Proteins are crucial for tissue repair post-surgery or injury.
- Malnutrition Management – Nurses must identify and manage protein-energy malnutrition (PEM) like kwashiorkor and marasmus.
- Critical Care Nutrition – Patients in ICU, burn units, or recovering from infections require high protein intake.
- Geriatric Care – Elderly patients need adequate protein to prevent muscle wasting (sarcopenia).
- Maternal and Child Health – Pregnant and lactating women require increased protein intake for fetal growth and milk production.
Classification of Proteins –
Proteins are essential macronutrients that perform various structural, enzymatic, and regulatory functions in the body. Based on their structure, function, and composition, proteins can be classified into different categories.
1. Classification Based on Composition
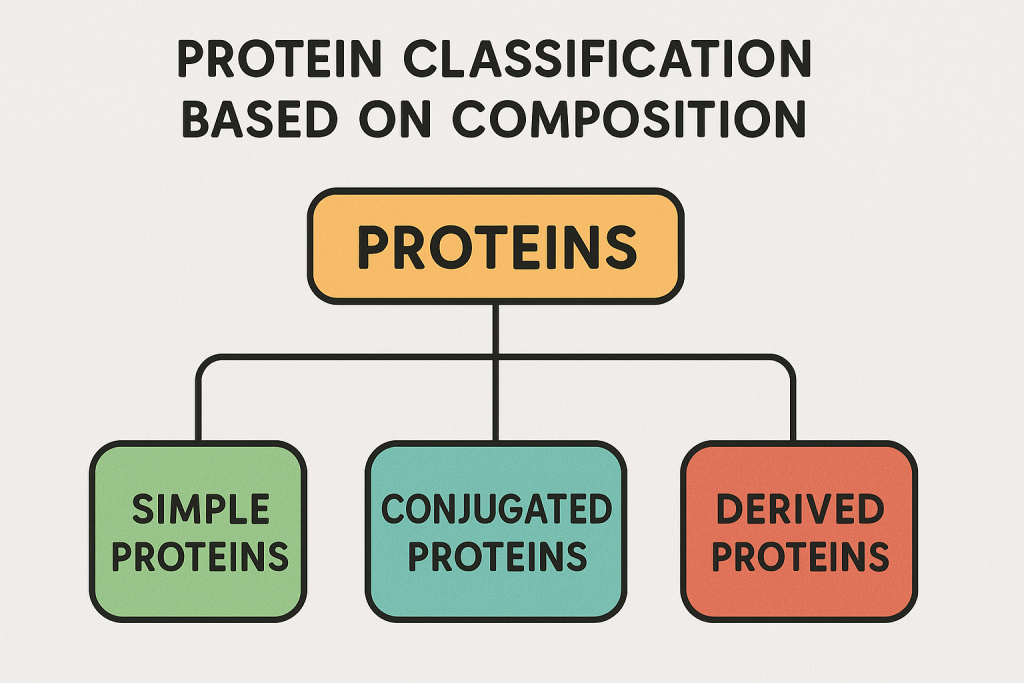
Proteins are classified into three main types based on their chemical composition:
A. Simple Proteins
- Composed only of amino acids.
- Yield only amino acids upon hydrolysis.
- Examples:
- Albumins (egg white, blood plasma)
- Globulins (antibodies, muscle proteins)
- Histones (found in DNA)
- Protamines (sperm cells)
- Glutelins (wheat gluten)
B. Conjugated Proteins
- Composed of amino acids plus a non-protein component (prosthetic group).
- The prosthetic group determines the function of the protein.
- Examples:
- Glycoproteins – Protein + Carbohydrate (e.g., mucin in mucus)
- Lipoproteins – Protein + Lipid (e.g., HDL, LDL in blood)
- Phosphoproteins – Protein + Phosphate (e.g., casein in milk)
- Hemoproteins – Protein + Iron (e.g., hemoglobin, myoglobin)
- Nucleoproteins – Protein + Nucleic Acid (e.g., ribosomes)
C. Derived Proteins
- Breakdown products of proteins formed during digestion or metabolism.
- Examples:
- Peptones – Intermediate breakdown products of proteins.
- Proteoses – Partially digested proteins.
- Polypeptides – Short chains of amino acids.
2. Classification Based on Structure
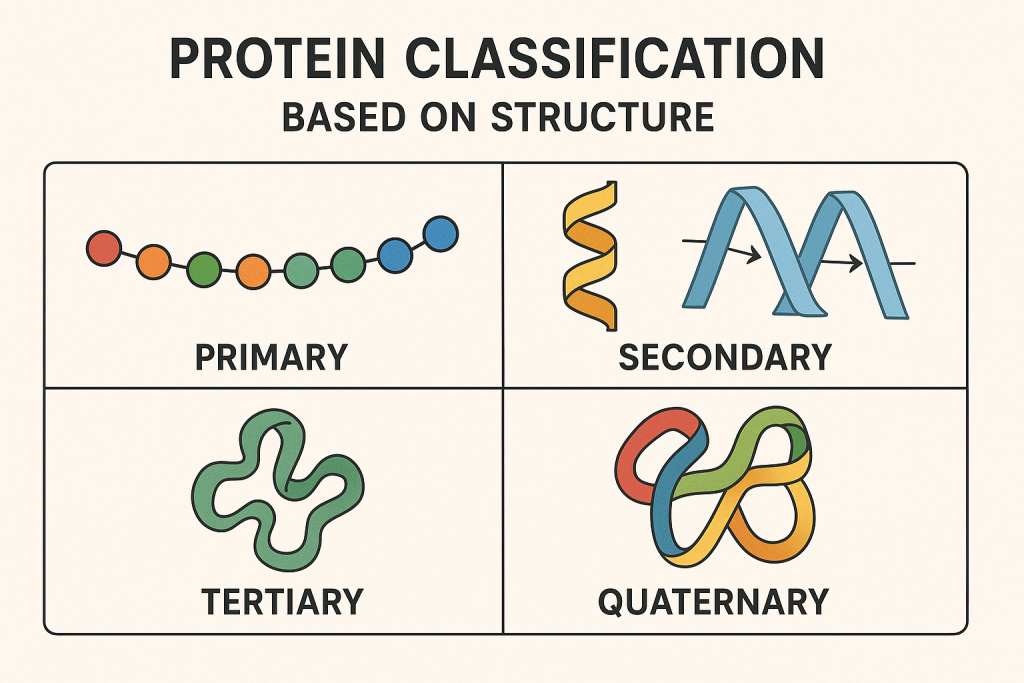
Proteins can be classified into two main structural types:
A. Fibrous Proteins
- Long, insoluble, and structural in function.
- Provide mechanical support and strength.
- Examples:
- Collagen – Found in connective tissue, bones, skin.
- Keratin – Found in hair, nails, and outer skin.
- Elastin – Provides elasticity in ligaments and skin.
- Myosin – Found in muscle fibers.
B. Globular Proteins
- Spherical, soluble in water, and perform dynamic functions.
- Examples:
- Enzymes (e.g., amylase, pepsin)
- Hormones (e.g., insulin, growth hormone)
- Hemoglobin – Oxygen transport in blood.
- Immunoglobulins – Antibodies in immune response.
3. Classification Based on Function
Proteins are classified based on their role in the body:
| Type of Protein | Function | Examples |
|---|---|---|
| Structural Proteins | Provide support and shape | Collagen, Keratin |
| Enzymatic Proteins | Catalyze biochemical reactions | Amylase, Pepsin |
| Transport Proteins | Carry molecules across the body | Hemoglobin, Albumin |
| Hormonal Proteins | Regulate metabolic processes | Insulin, Growth Hormone |
| Immune Proteins | Defend against infections | Immunoglobulins (Antibodies) |
| Contractile Proteins | Muscle contraction and movement | Actin, Myosin |
| Storage Proteins | Store essential molecules | Ferritin (Iron), Casein (Milk Protein) |
4. Classification Based on Nutritional Value
Proteins are classified based on their ability to provide essential amino acids.
A. Complete Proteins
- Contain all essential amino acids in the right proportion.
- Mostly from animal sources.
- Examples:
- Meat, Fish, Eggs, Milk, Cheese, Soybeans, Quinoa.
B. Incomplete Proteins
- Lack one or more essential amino acids.
- Mostly from plant sources.
- Examples:
- Legumes, Grains, Nuts, Vegetables.
C. Complementary Proteins
- A combination of two or more incomplete proteins that together provide all essential amino acids.
- Examples:
- Rice + Beans
- Wheat + Peanut Butter
- Corn + Lentils
5. Classification Based on Solubility
Proteins are categorized based on their solubility in different solvents.
| Type of Protein | Solubility | Examples |
|---|---|---|
| Albumins | Soluble in water | Egg albumin, Serum albumin |
| Globulins | Insoluble in pure water, soluble in salt solutions | Immunoglobulins, Myosin |
| Glutelins | Soluble in dilute acids/alkalis | Gluten in wheat |
| Prolamins | Soluble in alcohol | Zein in corn, Gliadin in wheat |
| Scleroproteins | Insoluble in water, tough structural proteins | Collagen, Kerati |
6. Classification Based on Source
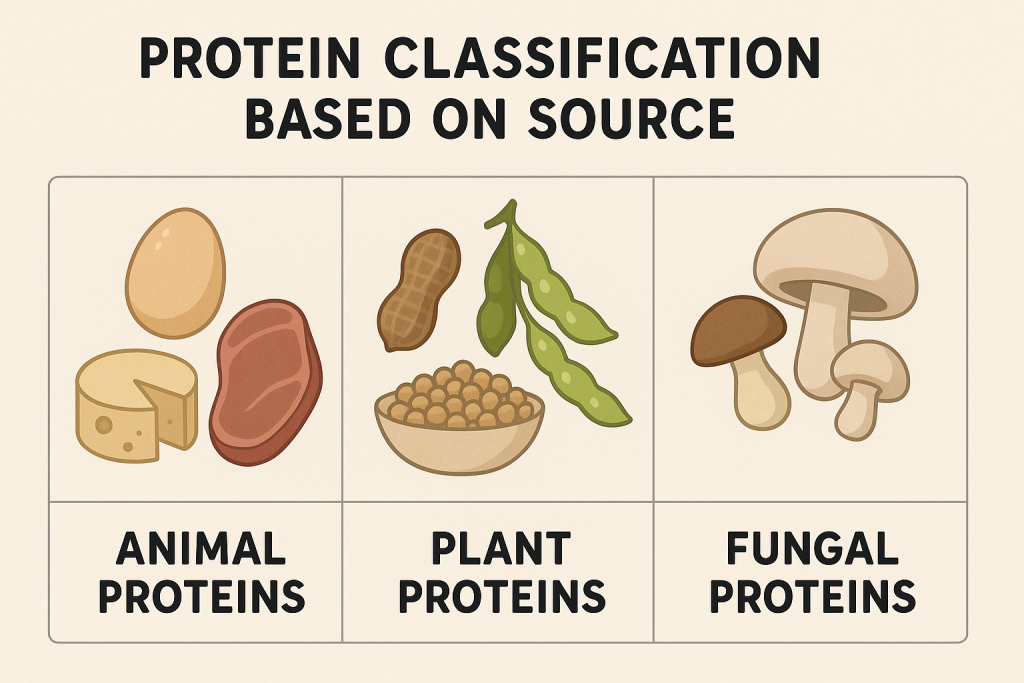
Proteins are classified based on their origin.
A. Animal Proteins
- High biological value.
- Rich in essential amino acids.
- Examples:
- Meat, Poultry, Fish, Eggs, Milk, Cheese.
B. Plant Proteins
- Lower biological value but rich in fiber and phytochemicals.
- Examples:
- Beans, Lentils, Chickpeas, Tofu, Nuts, Whole Grains.
7. Classification Based on Biological Value (BV)
Biological value (BV) refers to the percentage of absorbed protein that is utilized for body growth and maintenance.
A. High Biological Value (HBV) Proteins
- Provide all essential amino acids in the right proportions.
- Examples:
- Eggs (BV = 100)
- Milk (BV = 90)
- Meat & Fish (BV = 80-90)
- Soy Protein (BV = 74)
B. Low Biological Value (LBV) Proteins
- Deficient in one or more essential amino acids.
- Examples:
- Wheat (BV = 60)
- Rice (BV = 55)
- Beans & Lentils (BV = 50)
8. Classification Based on Protein Quality
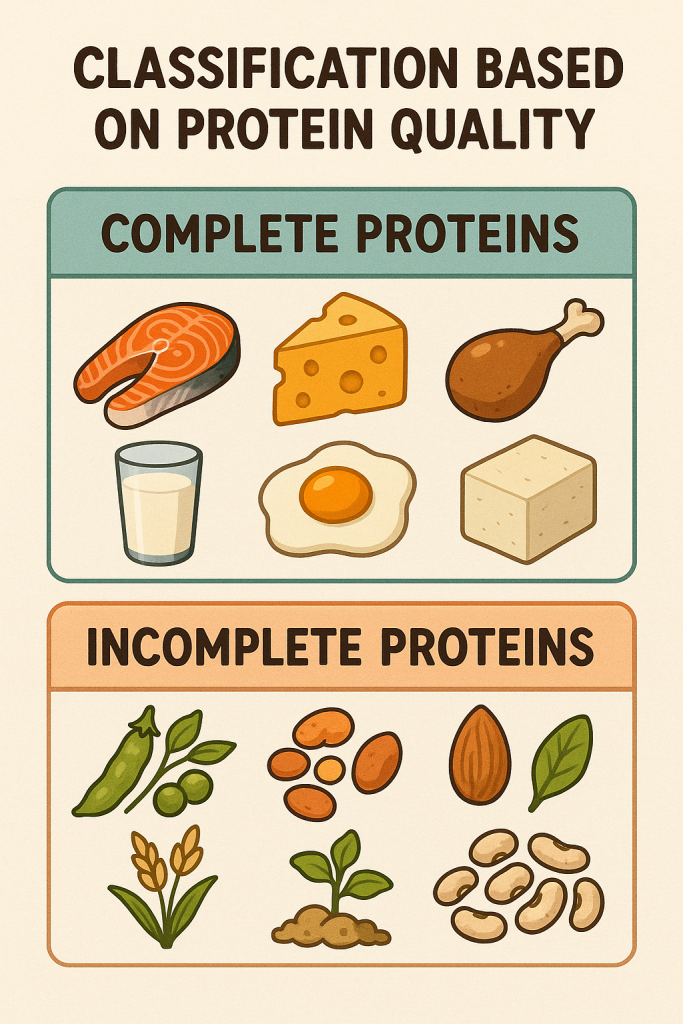
A. Complete Proteins
- Contain all essential amino acids.
- Examples: Egg, Fish, Milk.
B. Partially Complete Proteins
- Support maintenance but not growth.
- Examples: Gelatin.
C. Incomplete Proteins
- Cannot support maintenance or growth alone.
- Examples: Zein in Corn.
9. Functional Properties of Proteins
Proteins have various properties that determine their role in food and biological systems.
| Property | Function |
|---|---|
| Gel Formation | Forms gels when heated and cooled (e.g., gelatin in jelly). |
| Foaming | Creates foams in whipped cream and beaten egg whites. |
| Water Absorption | Retains moisture in food. |
| Emulsification | Helps in fat dispersion (e.g., egg yolk in mayonnaise). |
| Coagulation | Proteins coagulate when heated (e.g., curdling of milk). |
Eight Essential Amino Acids
Introduction
Amino acids are the building blocks of proteins, essential for various physiological functions such as growth, repair, and enzyme production. Among the 20 amino acids required by the human body, eight are classified as essential amino acids because they cannot be synthesized by the body and must be obtained through diet.
List of Eight Essential Amino Acids
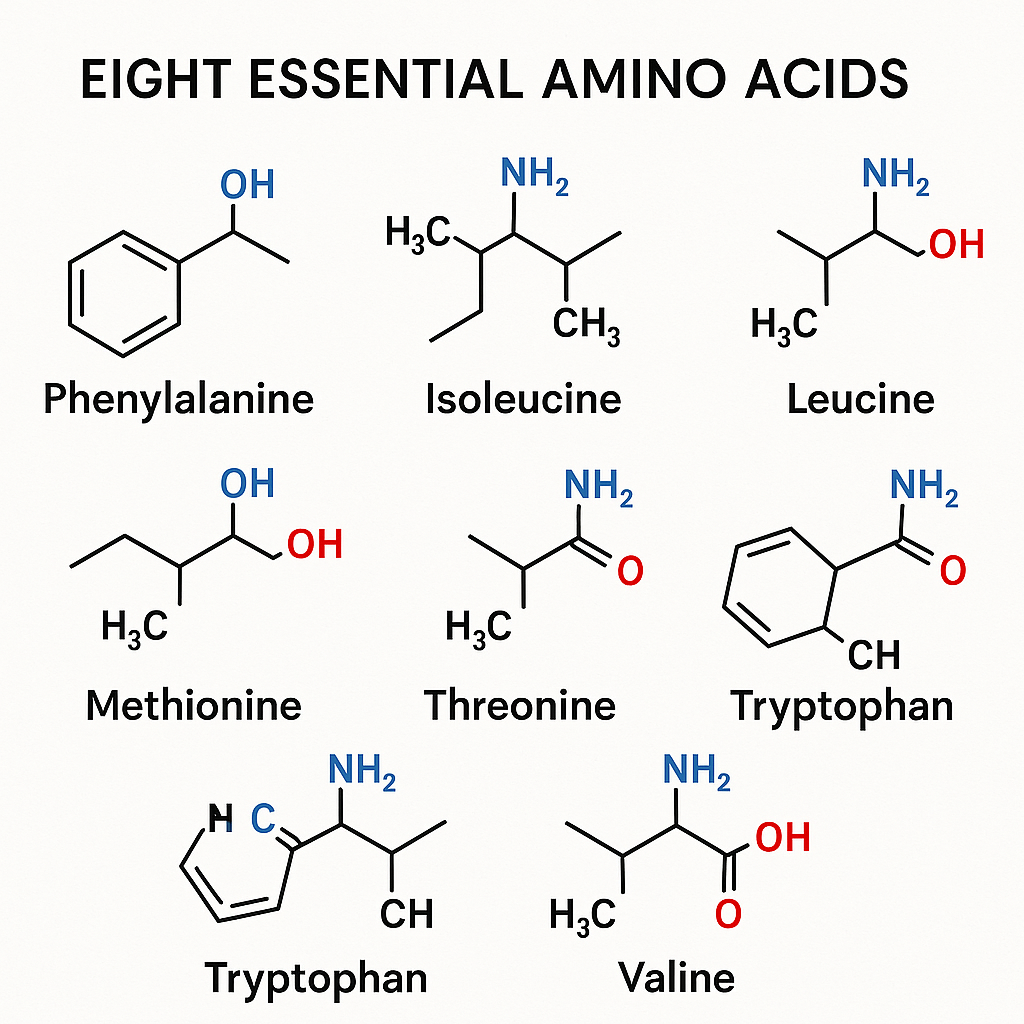
The eight essential amino acids are:
- Phenylalanine
- Valine
- Threonine
- Tryptophan
- Methionine
- Leucine
- Isoleucine
- Lysine
(Mnemonic: PVT TIM LL)
These amino acids are critical for protein synthesis, tissue growth, neurotransmitter function, and various metabolic processes.
Detailed Functions and Sources of Each Essential Amino Acid
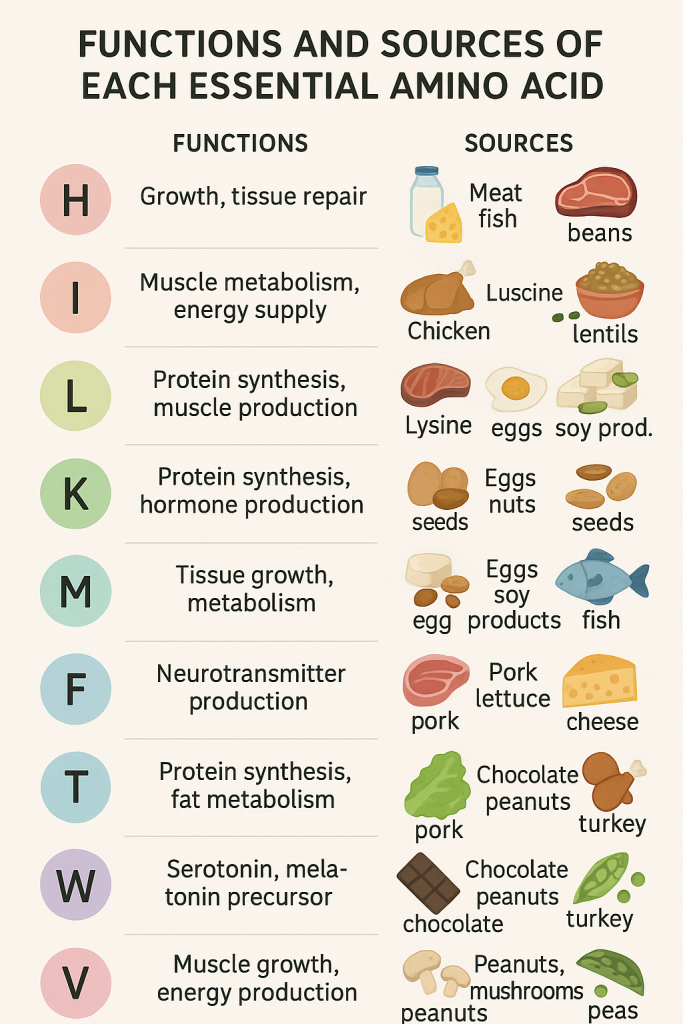
1. Phenylalanine (Phe)
Functions:
- Precursor for neurotransmitters dopamine, epinephrine, and norepinephrine.
- Essential for the production of thyroid hormones.
- Plays a role in pain relief and mood regulation.
Dietary Sources:
- Meat (beef, chicken, fish)
- Eggs
- Dairy products (cheese, milk, yogurt)
- Soybeans
- Nuts and seeds (almonds, sunflower seeds)
Deficiency Symptoms:
- Memory loss
- Depression and mood disorders
- Skin disorders like eczema
2. Valine (Val)
Functions:
- Helps in muscle metabolism and growth.
- Involved in energy production.
- Plays a role in tissue repair.
Dietary Sources:
- Meat (beef, poultry)
- Dairy products (cheese, yogurt)
- Peanuts and soy products
- Whole grains (brown rice, oats)
Deficiency Symptoms:
- Muscle weakness
- Fatigue
- Poor concentration
3. Threonine (Thr)
Functions:
- Helps in the formation of collagen and elastin (important for skin and connective tissues).
- Supports immune function.
- Essential for fat metabolism in the liver.
Dietary Sources:
- Meat and poultry
- Dairy products
- Nuts and seeds
- Leafy green vegetables
Deficiency Symptoms:
- Fatty liver
- Impaired immune function
- Digestive disorders
4. Tryptophan (Trp)
Functions:
- Precursor for serotonin and melatonin (important for mood regulation and sleep).
- Plays a role in niacin (Vitamin B3) production.
- Helps in reducing stress and anxiety.
Dietary Sources:
- Turkey and chicken
- Fish
- Dairy products
- Nuts and seeds
- Chocolate and bananas
Deficiency Symptoms:
- Depression and anxiety
- Sleep disturbances (insomnia)
- Poor appetite
5. Methionine (Met)
Functions:
- Involved in detoxification and metabolism.
- A precursor for cysteine and glutathione (important antioxidants).
- Supports hair and nail growth.
Dietary Sources:
- Meat and fish
- Dairy products
- Eggs
- Nuts and seeds
- Beans and lentils
Deficiency Symptoms:
- Fatty liver
- Slow wound healing
- Weak immune system
6. Leucine (Leu)
Functions:
- Plays a critical role in muscle growth and repair.
- Helps in wound healing.
- Regulates blood sugar levels.
Dietary Sources:
- Red meat
- Dairy products
- Peanuts
- Lentils and chickpeas
- Whole wheat products
Deficiency Symptoms:
- Muscle wasting
- Fatigue
- Low energy levels
7. Isoleucine (Ile)
Functions:
- Involved in muscle metabolism and immune function.
- Helps in hemoglobin production.
- Regulates blood sugar levels.
Dietary Sources:
- Meat, fish, and poultry
- Dairy products
- Nuts and seeds
- Whole grains
Deficiency Symptoms:
- Muscle weakness
- Dizziness and confusion
- Poor immune function
8. Lysine (Lys)
Functions:
- Essential for collagen formation (important for bones and skin).
- Helps in calcium absorption.
- Supports immune function and antibody production.
Dietary Sources:
- Red meat and poultry
- Fish (cod, sardines)
- Dairy products
- Legumes (beans, peas)
- Quinoa
Deficiency Symptoms:
- Poor immune function
- Fatigue and dizziness
- Hair loss
Importance of Essential Amino Acids in Nursing and Healthcare
- Growth and Development – Essential for children, pregnant women, and recovering patients.
- Muscle Maintenance – Helps prevent muscle atrophy in bedridden patients.
- Immune System Support – Essential for fighting infections and diseases.
- Wound Healing – Crucial in post-surgical recovery and burn patients.
- Mental Health – Supports neurotransmitter production, reducing stress and anxiety.
Deficiency of Essential Amino Acids
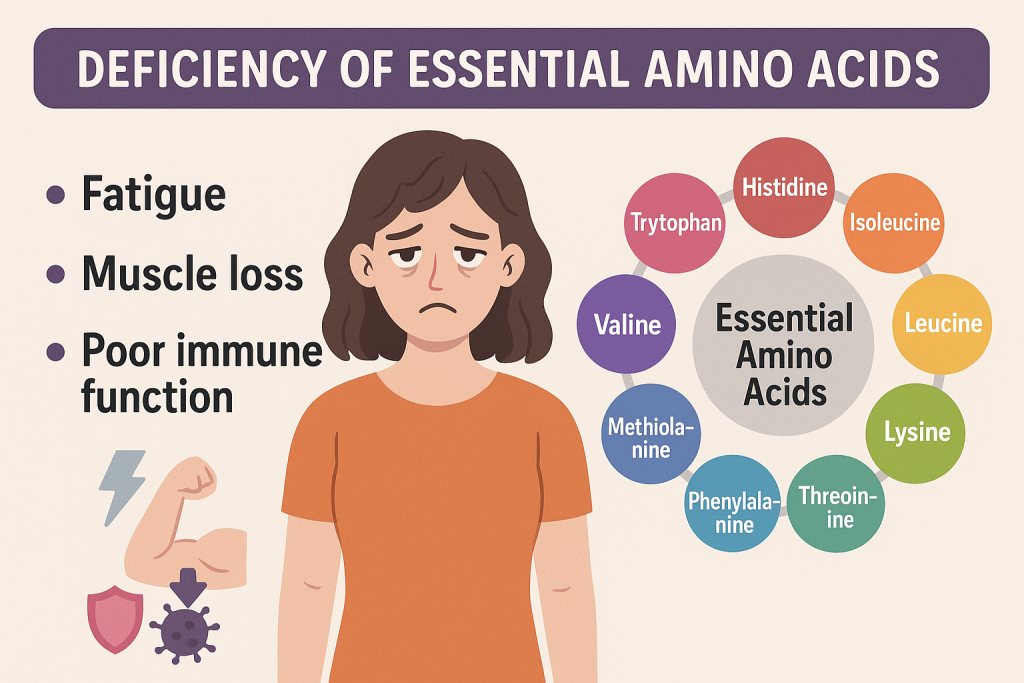
If a person does not consume enough essential amino acids, it can lead to:
- Growth retardation in children
- Muscle loss and weakness
- Poor immune function
- Neurological issues such as confusion, depression, and anxiety
- Fatty liver and metabolic disorders
Sources of Essential Amino Acids
To ensure adequate intake, one should consume:
- Animal proteins (Complete Proteins): Meat, fish, eggs, dairy.
- Plant-based proteins (Complementary Proteins):
- Legumes + Grains (e.g., rice and beans)
- Nuts + Whole Grains (e.g., peanut butter on whole wheat bread)
Dietary Sources of Protein
Protein is an essential macronutrient required for growth, repair, and maintenance of body tissues. It is found in both animal-based and plant-based foods, each providing different types of amino acids.
1. Animal-Based Sources of Protein (Complete Proteins)
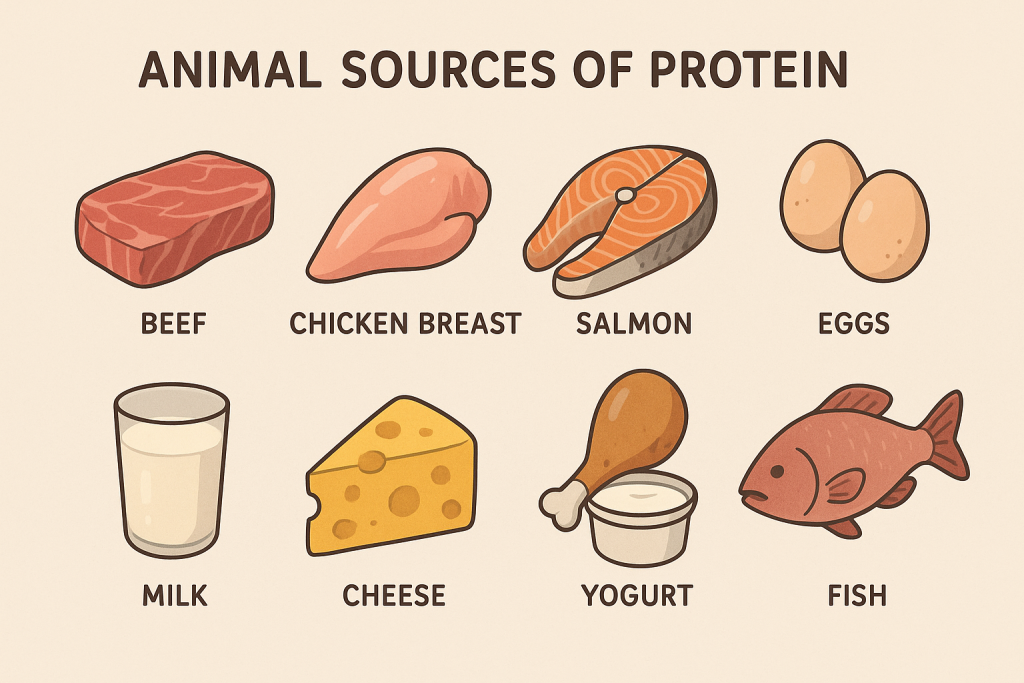
Animal proteins are considered complete proteins because they provide all essential amino acids in the right proportions.
A. Meat and Poultry
- Chicken (100g = 27g protein)
- Turkey (100g = 29g protein)
- Beef (100g = 26g protein)
- Pork (100g = 25g protein)
- Lamb (100g = 25g protein)
B. Fish and Seafood
- Salmon (100g = 25g protein)
- Tuna (100g = 30g protein)
- Cod (100g = 20g protein)
- Shrimp (100g = 24g protein)
- Crab (100g = 19g protein)
C. Dairy Products
- Milk (1 cup = 8g protein)
- Cheese (Cheddar, Mozzarella, Cottage Cheese) (100g = 22–30g protein)
- Yogurt (1 cup = 10g protein)
- Butter (Low in protein but contains essential fats)
D. Eggs
- Whole egg (1 large = 6g protein)
- Egg whites (1 large = 3.5g protein)
2. Plant-Based Sources of Protein (Incomplete Proteins)
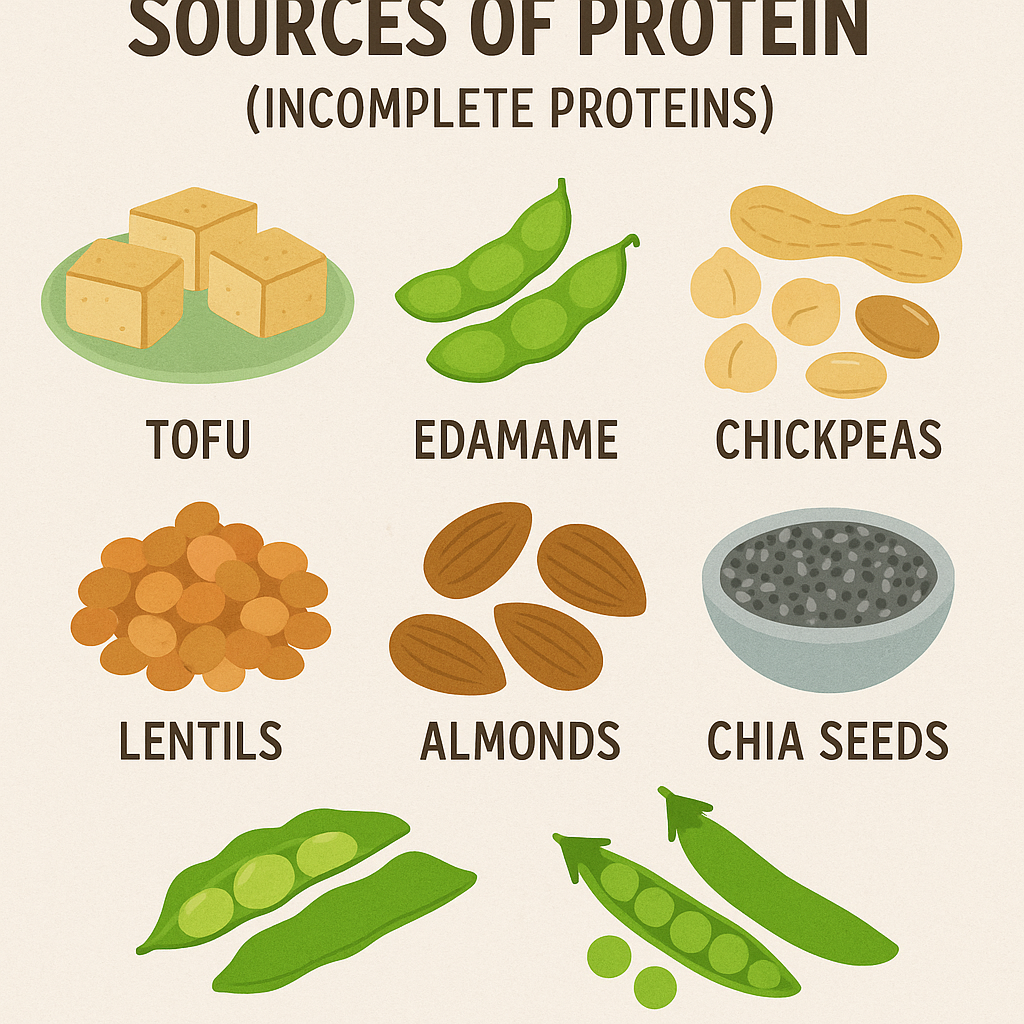
Plant-based proteins often lack one or more essential amino acids but can be combined to form complete proteins.
A. Legumes and Pulses
- Lentils (100g cooked = 9g protein)
- Chickpeas (Garbanzo Beans) (100g cooked = 8g protein)
- Black Beans (100g cooked = 9g protein)
- Kidney Beans (100g cooked = 8g protein)
- Green Peas (100g cooked = 5g protein)
B. Nuts and Seeds
- Almonds (100g = 21g protein)
- Peanuts (100g = 26g protein)
- Walnuts (100g = 15g protein)
- Chia Seeds (100g = 17g protein)
- Flaxseeds (100g = 18g protein)
- Sunflower Seeds (100g = 21g protein)
- Pumpkin Seeds (100g = 19g protein)
C. Whole Grains
- Quinoa (100g cooked = 4g protein) (Complete protein)
- Brown Rice (100g cooked = 3g protein)
- Oats (100g cooked = 6g protein)
- Whole Wheat Bread (1 slice = 3–5g protein)
- Corn (100g = 3g protein)
D. Soy Products (Complete Plant Proteins)
- Tofu (100g = 8g protein)
- Tempeh (100g = 19g protein)
- Soy Milk (1 cup = 7g protein)
- Edamame (Young Soybeans) (100g = 11g protein)
E. Vegetables (Low but Important Protein Sources)
- Spinach (100g cooked = 3g protein)
- Broccoli (100g cooked = 2.8g protein)
- Mushrooms (100g = 3g protein)
- Brussels Sprouts (100g = 3g protein)
- Asparagus (100g = 2g protein)
3. Protein Supplements
For individuals who need additional protein intake, supplements can be used.
A. Protein Powders
- Whey Protein (High in leucine, used for muscle building)
- Casein Protein (Slow-digesting protein, good for nighttime use)
- Soy Protein (Plant-based alternative, good for vegetarians)
- Pea Protein (Hypoallergenic, suitable for people with allergies)
- Rice Protein (Lower in lysine but still beneficial)
4. Comparison of Protein Content in Different Sources
| Food Item | Protein per 100g |
|---|---|
| Chicken Breast | 27g |
| Beef | 26g |
| Salmon | 25g |
| Tuna | 30g |
| Eggs (whole) | 6g (per egg) |
| Milk | 8g (per cup) |
| Cheese (Cheddar) | 25g |
| Lentils | 9g |
| Chickpeas | 8g |
| Tofu | 8g |
| Almonds | 21g |
| Peanuts | 26g |
| Quinoa | 4g |
| Broccoli | 2.8g |
5. Combining Plant-Based Proteins for a Complete Amino Acid Profile
Since most plant-based proteins are incomplete proteins, they should be combined to ensure all essential amino acids are consumed.
| Food Combination | Example Dishes |
|---|---|
| Legumes + Grains | Rice and Beans, Dal with Roti |
| Grains + Nuts/Seeds | Oatmeal with Almonds, Bread & Peanut Butter |
| Vegetables + Seeds | Spinach Salad with Sunflower Seeds |
| Soy + Whole Grains | Tofu with Brown Rice |
6. Choosing the Right Protein Sources for Different Diets
| Diet Type | Best Protein Sources |
|---|---|
| Vegetarian | Dairy, eggs, legumes, nuts, seeds, quinoa, soy products |
| Vegan | Legumes, soy, nuts, seeds, whole grains, vegetables |
| Keto Diet | Meat, fish, eggs, cheese, nuts, low-carb vegetables |
| Weight Loss | Lean meats, fish, egg whites, legumes, soy products |
| Muscle Building | Chicken, beef, fish, eggs, dairy, whey protein |
7. Protein Requirements for Different Age Groups
| Category | Recommended Daily Allowance (RDA) |
|---|---|
| Infants (0-1 year) | 1.5 g/kg body weight |
| Children (1-10 years) | 1.0 g/kg body weight |
| Adolescents (11-18 years) | 0.9 g/kg body weight |
| Adults | 0.8 g/kg body weight |
| Pregnant Women | 1.1 g/kg body weight |
| Lactating Women | 1.3 g/kg body weight |
| Athletes & Bodybuilders | 1.2 – 2.0 g/kg body weight |
Functions of Proteins
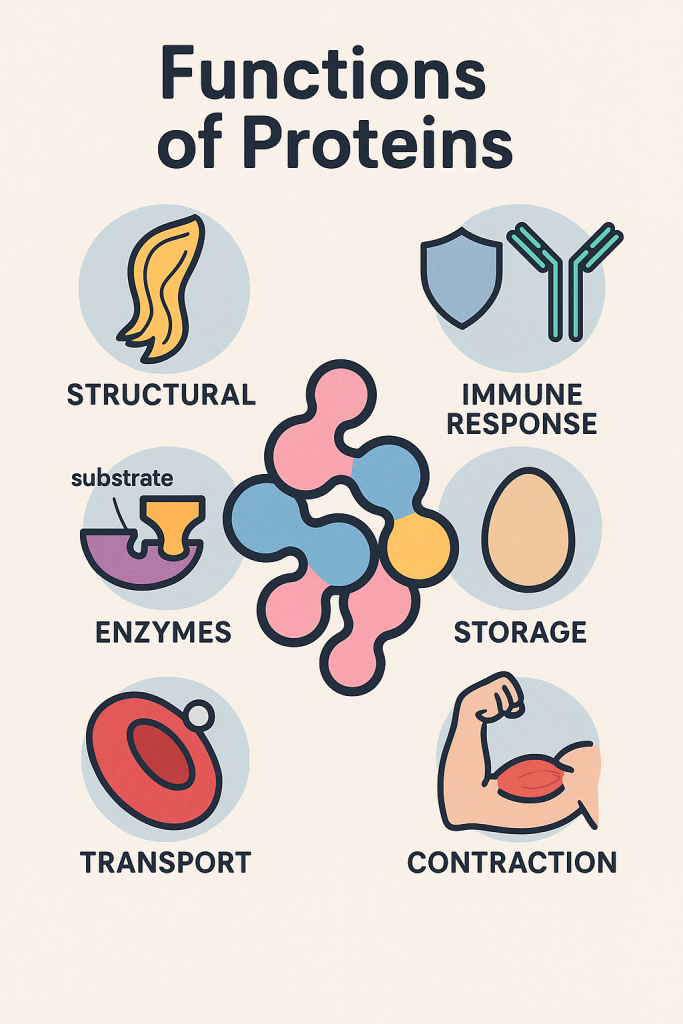
Proteins play a crucial role in the human body, performing various structural, regulatory, and metabolic functions. Since proteins are made up of amino acids, they serve as building blocks for different body tissues and biological processes.
1. Structural Functions
Proteins are essential for the structure and strength of body tissues.
A. Body Tissue Formation
- Proteins provide structural support to muscles, bones, skin, hair, and nails.
- Example: Collagen (found in connective tissues) provides strength to bones, skin, and tendons.
B. Growth and Development
- Necessary for growth during childhood, adolescence, pregnancy, and muscle repair.
- Example: Keratin strengthens hair, nails, and the outer layer of skin.
2. Enzymatic Functions
Enzymes are specialized proteins that catalyze biochemical reactions, speeding up metabolism.
- Example: Pepsin (digests proteins in the stomach).
- Example: Amylase (breaks down carbohydrates into sugars).
Without enzymes, metabolic processes would be too slow to sustain life.
3. Transport and Storage Functions
Proteins help in the transportation and storage of important molecules in the body.
A. Transport Proteins
- Hemoglobin transports oxygen from the lungs to tissues.
- Albumin carries hormones, fatty acids, and drugs in the blood.
- Transferrin transports iron to different body tissues.
B. Storage Proteins
- Ferritin stores iron in the liver.
- Casein stores protein in milk for infant nutrition.
4. Immune Functions (Defense Mechanism)
Proteins play a key role in the body’s immune system by forming antibodies.
- Immunoglobulins (Antibodies) help fight infections by identifying and neutralizing bacteria, viruses, and toxins.
- Complement Proteins enhance immune response.
Without proteins, the body would be unable to defend itself against diseases.
5. Hormonal Functions (Regulatory Role)
Proteins help regulate bodily functions by producing hormones.
- Insulin regulates blood sugar levels.
- Thyroxine regulates metabolism.
- Growth Hormone stimulates body growth and cell regeneration.
Protein-based hormones ensure homeostasis and proper organ function.
6. Contractile and Movement Functions
Proteins play an essential role in muscle contraction and body movement.
- Actin and Myosin in muscle fibers enable muscle contraction.
- Help in movements like walking, breathing, and heartbeat.
Without proteins, muscle strength and mobility would be compromised.
7. Energy Source
Proteins act as an energy reserve when carbohydrates and fats are insufficient.
- 1 gram of protein provides 4 kcal of energy.
- During starvation, the body breaks down muscle proteins for energy.
However, excess protein consumption for energy may lead to kidney strain and dehydration.
8. Buffering Function (Acid-Base Balance)
Proteins help maintain the pH balance in the body.
- Hemoglobin in the blood acts as a buffer to maintain pH.
- Plasma proteins (e.g., albumin) prevent excessive acidity or alkalinity.
Without this function, acidic blood conditions (acidosis) or alkaline conditions (alkalosis) could occur, leading to organ damage.
9. Fluid Balance Maintenance
Proteins regulate osmotic pressure, preventing excess fluid loss from the blood.
- Albumin and Globulin maintain blood volume and fluid distribution.
- Prevents conditions like edema (swelling due to fluid retention).
A lack of protein leads to malnutrition-related edema, seen in Kwashiorkor.
10. Wound Healing and Tissue Repair
Proteins help repair tissues and heal wounds by generating new cells.
- Fibrinogen helps in blood clotting.
- Collagen helps in wound healing and skin regeneration.
Patients recovering from surgery, burns, or injuries require high protein intake.
11. Reproductive and Fetal Development
Proteins are essential for fetal growth and milk production during pregnancy and lactation.
- Casein in breast milk supports infant growth.
- Hormones like prolactin regulate milk production.
Pregnant and lactating women need higher protein intake to support both mother and baby.
Summary Table: Functions of Proteins
| Function | Examples |
|---|---|
| Structural Support | Collagen, Keratin |
| Growth & Development | Muscle, Hair, Skin |
| Enzymatic Reactions | Pepsin, Amylase |
| Transport | Hemoglobin (Oxygen), Albumin (Nutrients) |
| Storage | Ferritin (Iron), Casein (Milk) |
| Immune Defense | Immunoglobulins (Antibodies) |
| Hormone Regulation | Insulin, Growth Hormone |
| Muscle Contraction | Actin, Myosin |
| Energy Source | Used when carbs/fats are low |
| Acid-Base Balance | Hemoglobin, Plasma Proteins |
| Fluid Balance | Albumin (Prevents Edema) |
| Wound Healing | Fibrinogen (Clotting), Collagen |
| Reproductive Functions | Prolactin (Lactation) |
Protein Requirements – Recommended Dietary Allowance (RDA)
Introduction
Protein is an essential macronutrient required for growth, repair, immune function, enzyme production, and overall metabolism. The Recommended Dietary Allowance (RDA) for protein varies based on age, sex, physiological condition, and activity level. The RDA is expressed as grams of protein per kilogram (g/kg) of body weight per day.
1. Protein Requirements Based on Age and Gender
A. Infants and Children
Protein is crucial for growth and development in infants and children.
| Age Group | RDA (g/kg body weight/day) | Total Daily Requirement |
|---|---|---|
| 0 – 6 months | 1.5 g/kg | 9–15g (Based on average weight) |
| 6 – 12 months | 1.2 g/kg | 11–18g |
| 1 – 3 years | 1.05 g/kg | 13g |
| 4 – 8 years | 0.95 g/kg | 19g |
| 9 – 13 years | 0.85 g/kg | 34g |
B. Adolescents
Adolescents require higher protein intake due to rapid growth and hormonal changes.
| Age Group | RDA (g/kg body weight/day) | Total Daily Requirement |
|---|---|---|
| Boys (14–18 years) | 0.85 g/kg | 52g |
| Girls (14–18 years) | 0.85 g/kg | 46g |
C. Adults
Adult protein requirements are based on body maintenance and metabolism.
| Age Group | RDA (g/kg body weight/day) | Total Daily Requirement |
|---|---|---|
| Men (19–50 years) | 0.8 g/kg | 56g |
| Women (19–50 years) | 0.8 g/kg | 46g |
| Men (51+ years) | 0.8 g/kg | 56g |
| Women (51+ years) | 0.8 g/kg | 46g |
2. Protein Requirements for Special Groups
A. Pregnant and Lactating Women
Pregnant and breastfeeding women require additional protein for fetal growth, milk production, and maternal health.
| Condition | RDA (g/kg body weight/day) | Total Daily Requirement |
|---|---|---|
| Pregnancy (1st trimester) | 0.8 g/kg | 46g |
| Pregnancy (2nd & 3rd trimester) | 1.1 g/kg | 71g |
| Lactation (0–6 months) | 1.3 g/kg | 71g |
| Lactation (6–12 months) | 1.3 g/kg | 71g |
B. Elderly Individuals
Older adults require higher protein intake to prevent muscle loss (sarcopenia) and maintain immune function.
| Age Group | RDA (g/kg body weight/day) | Total Daily Requirement |
|---|---|---|
| 60+ years | 1.0 – 1.2 g/kg | 50–75g |
C. Athletes and Bodybuilders
Athletes need more protein for muscle repair, endurance, and strength building.
| Activity Level | RDA (g/kg body weight/day) |
|---|---|
| Sedentary person | 0.8 g/kg |
| Endurance athletes | 1.2 – 1.4 g/kg |
| Strength/Power athletes | 1.6 – 2.0 g/kg |
| Bodybuilders (Muscle gain phase) | 1.6 – 2.2 g/kg |
3. Protein Requirements Based on Body Weight
Protein needs can be calculated using body weight. The formula:
Protein Requirement (g) = Body Weight (kg) × RDA (g/kg body weight)
Examples of Daily Protein Needs:
| Body Weight | Sedentary (0.8 g/kg) | Athlete (1.5 g/kg) | Bodybuilder (2.0 g/kg) |
|---|---|---|---|
| 50 kg | 40g | 75g | 100g |
| 60 kg | 48g | 90g | 120g |
| 70 kg | 56g | 105g | 140g |
| 80 kg | 64g | 120g | 160g |
4. Effects of Protein Deficiency and Excess
A. Protein Deficiency (Hypoproteinemia)
Lack of sufficient protein intake leads to:
- Growth retardation (in children)
- Muscle wasting (loss of lean body mass)
- Edema (fluid retention, seen in Kwashiorkor)
- Weakened immunity (frequent infections)
- Fatty liver (due to poor metabolism)
B. Excess Protein Intake
Consuming excessive protein can cause:
- Kidney strain (excess nitrogen excretion)
- Dehydration (due to high urea production)
- Increased calcium loss (risk of osteoporosis)
- Weight gain (if excess protein is converted to fat)
5. Best Dietary Sources of Protein
A. Animal-Based Protein Sources (Complete Proteins)
- Chicken (100g = 27g protein)
- Fish (100g = 25g protein)
- Eggs (1 large = 6g protein)
- Milk (1 cup = 8g protein)
- Cheese (100g = 25g protein)
B. Plant-Based Protein Sources (Incomplete Proteins)
- Lentils (100g = 9g protein)
- Chickpeas (100g = 8g protein)
- Quinoa (100g = 4g protein – Complete Plant Protein)
- Tofu (100g = 8g protein)
- Nuts & Seeds (Almonds 100g = 21g protein)
6. How to Meet Daily Protein Requirements?
A. Balanced Meal Plan Example (For 60g Protein Intake)
| Meal | Food Item | Protein Content (g) |
|---|---|---|
| Breakfast | 2 eggs + whole wheat toast + 1 glass milk | 18g |
| Mid-Morning Snack | Handful of almonds | 6g |
| Lunch | Grilled chicken + brown rice + vegetables | 25g |
| Evening Snack | Yogurt with chia seeds | 8g |
| Dinner | Lentil soup + salad | 12g |
7. Adjusting Protein Intake for Special Cases
| Condition | Adjustment Needed |
|---|---|
| Kidney Disease | Reduce protein intake (0.6-0.8 g/kg) to prevent kidney overload |
| Liver Disease | Moderate protein intake (0.8-1.2 g/kg) to support liver function |
| Burn or Trauma Patients | Increase protein intake (1.5-2.0 g/kg) for tissue repair |
| Weight Loss | High-protein, low-calorie diet (1.2-1.6 g/kg) to preserve muscle mass |