BSC SEM 4 PHARMACOLOGY 2 UNIT 2 Drugs used on urinary system
UNIT 2 Drugs used on urinary system

Pharmacology of commonly used drugs Composition action dosage route indications contraindications Drug Interactions side effects adverse effects toxicity and role of nurse
Below is an overview of how pharmacology is typically approached for commonly used drugs, covering the key components such as composition, mechanism of action, dosage, route, indications, contraindications, interactions, side effects, adverse effects, toxicity, and the critical role of nurses in medication management.
1. Composition
- Definition:
The composition of a drug refers to its chemical makeup, including the active pharmaceutical ingredient (API) and any excipients (fillers, binders, preservatives, etc.). - Importance:
Knowing the composition helps determine the purity, stability, and potential allergenic properties of the drug.
2. Mechanism of Action (Action)
- Definition:
This is the process by which a drug produces its therapeutic effect at the cellular or molecular level. - Example:
- Acetaminophen (Paracetamol): It is thought to inhibit prostaglandin synthesis in the central nervous system, providing analgesic and antipyretic effects.
- Importance:
Understanding the mechanism helps in predicting therapeutic outcomes as well as potential interactions with other drugs.
3. Dosage and Route of Administration
- Dosage:
Refers to the amount of drug prescribed, including the frequency and duration of administration. For example, acetaminophen is typically dosed at 500–1000 mg every 4–6 hours, not exceeding 4 g per day. - Route:
Describes how the drug is administered—common routes include oral, intravenous (IV), intramuscular (IM), subcutaneous, topical, etc. - Importance:
Proper dosage and route ensure that the drug reaches its site of action in effective concentrations while minimizing the risk of toxicity.
4. Indications
- Definition:
These are the approved medical conditions or symptoms for which the drug is prescribed. - Example:
- Ibuprofen: Indicated for pain relief, inflammation reduction, and fever.
- Importance:
Clear indications guide clinicians in selecting the appropriate medication for a patient’s condition.
5. Contraindications
- Definition:
Conditions or factors that make the use of the drug inadvisable. - Example:
- Ibuprofen: Contraindicated in patients with active peptic ulcers or severe renal impairment.
- Importance:
Identifying contraindications prevents harm and avoids adverse reactions in susceptible individuals.
6. Drug Interactions
- Definition:
Interactions occur when the effect of one drug is altered by the presence of another substance (another drug, food, or supplement). - Example:
- Acetaminophen: Can interact with alcohol or warfarin, potentially increasing the risk of liver damage or bleeding.
- Importance:
Understanding interactions is critical to prevent reduced efficacy or enhanced toxicity.
7. Side Effects
- Definition:
These are usually mild, predictable, and often reversible unintended effects. - Example:
- Ibuprofen: May cause gastrointestinal upset or dizziness.
- Importance:
Monitoring and managing side effects help improve patient comfort and adherence to therapy.
8. Adverse Effects
- Definition:
More severe and sometimes unexpected reactions that can occur with drug use. - Example:
- Acetaminophen: Overdose can lead to significant hepatotoxicity.
- Importance:
Early identification and management are key to minimizing harm.
9. Toxicity
- Definition:
Toxicity refers to the harmful effects that occur when a drug is taken in excessive amounts, including both acute overdose and long-term exposure issues. - Example:
- Acetaminophen: Toxicity is primarily hepatotoxicity; prompt treatment with N-acetylcysteine is essential in overdose cases.
- Importance:
Knowledge of toxic doses and signs of toxicity is vital for timely intervention and prevention of serious complications.
10. Role of the Nurse
Nurses play an essential role in ensuring the safe and effective use of medications. Their responsibilities include:
- Administration:
Ensuring correct dosing, proper route, and timing of medication administration. - Monitoring:
Observing for both therapeutic effects and any signs of adverse reactions or toxicity. - Patient Education:
Explaining the purpose, proper usage, potential side effects, and what to do in case of a missed dose or overdose. - Assessment:
Regularly checking vital signs, lab values (e.g., liver function tests for acetaminophen), and overall patient condition to adjust therapy as needed. - Documentation:
Keeping accurate records of medication administration and any observed effects to inform the broader healthcare team. - Advocacy:
Being alert to any signs of drug interactions or contraindications, and communicating effectively with prescribers to ensure patient safety.
Renin angiotensin system
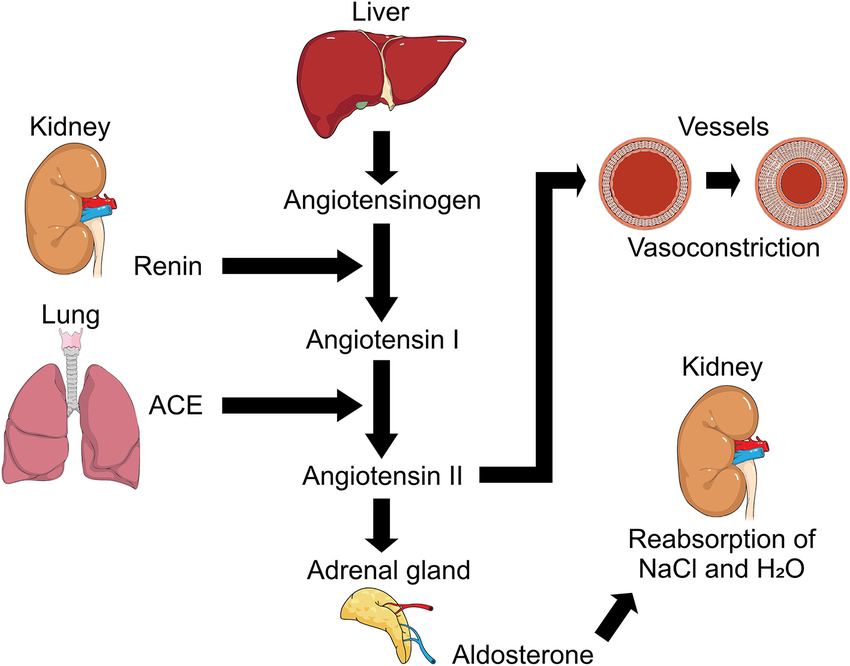
Below is a comprehensive overview of the renin–angiotensin system (RAS) as it relates to drugs targeting it. Although the RAS is a physiologic cascade rather than a single drug, many antihypertensive agents work by modulating this system. The following sections detail the components of the system and how pharmacologic agents—such as ACE inhibitors, ARBs, and direct renin inhibitors—are characterized in terms of composition, action, dosage, route, indications, contraindications, drug interactions, side effects, adverse effects, toxicity, and the role of the nurse.
1. Composition of the Renin–Angiotensin System
- Renin:
An enzyme produced by the juxtaglomerular cells of the kidney. It converts angiotensinogen (a liver-derived protein) into angiotensin I. - Angiotensinogen:
A precursor protein synthesized in the liver. - Angiotensin I:
An inactive decapeptide that is converted to angiotensin II by the angiotensin-converting enzyme (ACE). - Angiotensin-Converting Enzyme (ACE):
Primarily found in the pulmonary endothelium; it transforms angiotensin I into the active peptide angiotensin II. - Angiotensin II:
A potent vasoconstrictor that also stimulates aldosterone secretion, increases sympathetic activity, and promotes sodium and water retention. - Aldosterone:
A mineralocorticoid hormone released from the adrenal cortex, it enhances sodium reabsorption (and potassium excretion) in the kidneys.
2. Mechanism of Action (Pharmacologic Targeting)
Drugs that modulate the RAS work by interrupting this cascade, which in turn reduces vasoconstriction and fluid retention. For example:
- ACE Inhibitors (e.g., Lisinopril, Enalapril):
Inhibit the conversion of angiotensin I to angiotensin II, leading to vasodilation and decreased aldosterone-mediated sodium retention. - Angiotensin II Receptor Blockers (ARBs, e.g., Losartan, Valsartan):
Block the angiotensin II type 1 (AT1) receptor, preventing the vasoconstrictor and aldosterone-secreting effects of angiotensin II. - Direct Renin Inhibitors (e.g., Aliskiren):
Directly inhibit renin, thus reducing the formation of angiotensin I and downstream angiotensin II.
3. Dosage and Route
ACE Inhibitors
- Dosage:
- Lisinopril: Commonly initiated at 10 mg once daily, with adjustments up to 20–40 mg daily based on blood pressure response.
- Route:
- Oral administration.
ARBs
- Dosage:
- Losartan: Typically started at 50 mg once daily, with a maintenance dose of up to 100 mg/day.
- Route:
- Oral administration.
Direct Renin Inhibitors
- Dosage:
- Aliskiren: Usually started at 150 mg once daily and may be increased to 300 mg/day if needed.
- Route:
- Oral administration.
4. Indications
Drugs that act on the RAS are primarily indicated for:
- Hypertension:
They are widely used as first-line agents in managing high blood pressure. - Heart Failure:
Reduce afterload and prevent adverse cardiac remodeling. - Chronic Kidney Disease:
Particularly beneficial in slowing the progression of diabetic nephropathy. - Post-Myocardial Infarction:
Help reduce ventricular remodeling and improve survival outcomes.
5. Contraindications
ACE Inhibitors and ARBs
- History of Angioedema:
Patients with a prior history should generally avoid these drugs. - Bilateral Renal Artery Stenosis:
These agents can further reduce renal perfusion in this setting. - Pregnancy:
Use is contraindicated due to risks of fetal toxicity. - Hypersensitivity:
Known allergy to the specific agent.
Direct Renin Inhibitors
- Similar contraindications as ACE inhibitors/ARBs, including pregnancy and known hypersensitivity.
6. Drug Interactions
- Potassium-Sparing Diuretics/Potassium Supplements:
Concomitant use may lead to hyperkalemia. - Nonsteroidal Anti-Inflammatory Drugs (NSAIDs):
Can reduce the antihypertensive efficacy and may impair renal function. - Other Antihypertensives:
Combined use might necessitate dosage adjustments due to additive hypotensive effects. - Diuretics:
May enhance hypotension, particularly when starting therapy.
7. Side Effects
- ACE Inhibitors:
Common side effects include a persistent dry cough, hyperkalemia, and occasionally hypotension. - ARBs:
Typically cause fewer cough-related issues but may still lead to hyperkalemia and dizziness. - Direct Renin Inhibitors:
Side effects can include diarrhea, hypotension, and hyperkalemia.
8. Adverse Effects
- ACE Inhibitors:
Serious adverse effects include angioedema and, in rare cases, acute renal failure. - ARBs:
Severe hypotension or renal impairment may occur, though less frequently. - Direct Renin Inhibitors:
Adverse reactions are less common but require monitoring, especially regarding renal function and blood pressure.
9. Toxicity
- Overdose:
Overdosing on these agents may result in profound hypotension, shock, electrolyte imbalances, and acute kidney injury. - Management:
Immediate supportive care is essential, including fluid resuscitation and careful monitoring of renal function and electrolytes.
10. Role of the Nurse
Nurses are critical in the safe and effective administration of medications that target the RAS:
- Administration:
- Verify the correct dosage and route (typically oral).
- Ensure adherence to the prescribed medication schedule.
- Monitoring:
- Vital Signs: Regularly check blood pressure to assess therapeutic effectiveness.
- Laboratory Values: Monitor renal function (serum creatinine, eGFR) and electrolytes (especially potassium) to detect early signs of adverse effects.
- Clinical Signs: Watch for symptoms such as a persistent dry cough (common with ACE inhibitors) or signs of angioedema (swelling of the face, lips, or throat).
- Patient Education:
- Explain the purpose of the medication, expected benefits, and potential side effects.
- Advise on lifestyle modifications and the importance of medication adherence.
- Instruct patients to report any unusual symptoms (e.g., sudden swelling or severe dizziness) immediately.
- Documentation and Communication:
- Accurately record all observations, lab results, and patient-reported side effects.
- Communicate any concerns or significant changes in patient status to the prescribing physician for timely intervention.
Diuretics and antidiuretics
Below is a comprehensive overview of diuretics and antidiuretics, including their composition, mechanism of action, dosage and route, indications, contraindications, drug interactions, side effects, adverse effects, toxicity, and the nurse’s role in ensuring safe and effective use.
Diuretics

Diuretics are a class of medications that promote the excretion of water and electrolytes (primarily sodium) from the body. They are used to manage conditions such as hypertension, edema, and certain kidney disorders. Several subtypes exist, each with unique mechanisms and clinical applications.
1. Composition
- Loop Diuretics:
- Examples: Furosemide, Bumetanide
- Composition: Synthetic molecules that block the Na⁺-K⁺-2Cl⁻ cotransporter.
- Thiazide Diuretics:
- Examples: Hydrochlorothiazide, Chlorthalidone
- Composition: Benzothiadiazine derivatives that inhibit the Na⁺-Cl⁻ symporter.
- Potassium-Sparing Diuretics:
- Examples: Spironolactone, Eplerenone (aldosterone antagonists) and Amiloride (sodium channel blocker)
- Composition: Steroidal (spironolactone, eplerenone) or non-steroidal compounds (amiloride).
- Carbonic Anhydrase Inhibitors:
- Example: Acetazolamide
- Composition: Sulfonamide derivatives that inhibit carbonic anhydrase.
- Osmotic Diuretics:
- Example: Mannitol
- Composition: Sugar alcohols that increase the osmotic pressure in renal tubules.
2. Mechanism of Action
- Loop Diuretics:
Inhibit the Na⁺-K⁺-2Cl⁻ cotransporter in the thick ascending limb of the loop of Henle, leading to significant diuresis and reduction in fluid overload. - Thiazide Diuretics:
Block sodium and chloride reabsorption in the distal convoluted tubule, resulting in moderate diuresis and reduction in blood pressure. - Potassium-Sparing Diuretics:
Either antagonize aldosterone receptors in the collecting duct (spironolactone, eplerenone) or directly block sodium channels (amiloride), thereby conserving potassium while promoting sodium excretion. - Carbonic Anhydrase Inhibitors:
Inhibit the enzyme carbonic anhydrase, reducing bicarbonate reabsorption and causing a mild diuretic effect. - Osmotic Diuretics:
Increase the osmotic gradient in the nephron, drawing water into the tubular lumen and increasing urine output.
3. Dosage and Route
- Loop Diuretics (e.g., Furosemide):
- Dosage: Typically initiated at 20–80 mg orally; may require IV administration in acute settings.
- Route: Oral and intravenous.
- Thiazide Diuretics (e.g., Hydrochlorothiazide):
- Dosage: Often 12.5–50 mg once daily.
- Route: Oral.
- Potassium-Sparing Diuretics (e.g., Spironolactone):
- Dosage: Ranges from 25–100 mg daily, adjusted based on the clinical situation.
- Route: Oral.
- Carbonic Anhydrase Inhibitors (e.g., Acetazolamide):
- Dosage: Commonly 250–500 mg orally two to four times daily.
- Route: Oral and IV.
- Osmotic Diuretics (e.g., Mannitol):
- Dosage: Varies widely (e.g., 0.25–2 g/kg) depending on the indication.
- Route: Intravenous.
4. Indications
- Hypertension:
Diuretics (especially thiazides) lower blood pressure by reducing plasma volume. - Edema:
Used in congestive heart failure, renal failure, and hepatic cirrhosis to relieve fluid overload. - Cerebral Edema and Increased Intracranial Pressure:
Osmotic diuretics like mannitol are used. - Glaucoma:
Carbonic anhydrase inhibitors reduce intraocular pressure.
5. Contraindications
- General Contraindications:
- Severe dehydration or hypovolemia.
- Anuria (inability to produce urine).
- Electrolyte imbalances (e.g., severe hyponatremia or hypokalemia).
- Known hypersensitivity to the diuretic class.
- Specific Considerations:
- Loop diuretics are contraindicated in patients with significant renal impairment where they may exacerbate kidney injury.
- Thiazides may not be appropriate for patients with gout due to increased uric acid levels.
6. Drug Interactions
- Other Antihypertensives:
Enhanced hypotensive effects when combined with ACE inhibitors, ARBs, or beta-blockers. - NSAIDs:
Can diminish the diuretic and antihypertensive effects by causing sodium retention. - Other Electrolyte-Modifying Agents:
Concurrent use with other medications affecting potassium levels (e.g., potassium supplements or potassium-sparing diuretics) can result in significant imbalances.
7. Side Effects
- Common Side Effects:
- Electrolyte imbalances (e.g., hypokalemia with loop and thiazide diuretics; hyperkalemia with potassium-sparing agents).
- Dehydration and hypotension.
- Metabolic disturbances (e.g., hyperuricemia with thiazides, metabolic acidosis with carbonic anhydrase inhibitors).
8. Adverse Effects
- Severe Reactions:
- Ototoxicity (especially with high-dose loop diuretics).
- Significant renal dysfunction.
- Cardiac arrhythmias secondary to electrolyte disturbances.
- Allergic reactions in sulfa-allergic patients (common with many diuretics).
9. Toxicity
- Overdose:
Can lead to profound dehydration, electrolyte disturbances (severe hypokalemia or hyponatremia), hypotensive shock, and acute kidney injury. - Management:
Requires prompt supportive care, including fluid and electrolyte replacement and careful monitoring of renal function.
10. Role of the Nurse
- Administration:
Confirm correct dosage and route (oral vs. IV) and ensure adherence to timing and frequency. - Monitoring:
- Regularly check vital signs, especially blood pressure.
- Monitor fluid balance, daily weights, and intake/output charts.
- Assess electrolyte levels (potassium, sodium, magnesium) and renal function tests.
- Patient Education:
- Instruct patients on the importance of adherence, recognizing signs of dehydration, and when to seek help.
- Educate on dietary modifications (e.g., potassium-rich or low-potassium diets as appropriate).
- Documentation and Communication:
Record administration details, side effects, and any adverse reactions, and promptly report significant changes in patient status to the healthcare provider.
Antidiuretics

Antidiuretic agents work to reduce urine output by promoting water reabsorption in the kidneys. They are primarily used in conditions characterized by excessive diuresis, such as diabetes insipidus.
1. Composition
- Desmopressin (DDAVP):
A synthetic analogue of the naturally occurring antidiuretic hormone (ADH), vasopressin. - Vasopressin:
The endogenous hormone produced by the hypothalamus and released by the posterior pituitary.
2. Mechanism of Action
- Desmopressin:
Binds to V2 receptors in the renal collecting ducts, increasing the permeability of the tubular membrane to water and enhancing water reabsorption, thereby reducing urine output. - Vasopressin:
Exerts similar effects but is less commonly used pharmacologically due to its broader systemic actions.
3. Dosage and Route
- Desmopressin:
- Dosage: Varies with the condition; for central diabetes insipidus, it may range from 0.1 to 0.4 mg daily in divided doses.
- Route: Can be administered intranasally, orally, subcutaneously, or intravenously depending on the clinical scenario.
4. Indications
- Central Diabetes Insipidus:
To reduce polyuria and prevent dehydration. - Nocturnal Enuresis:
In some cases, to reduce nighttime urine production. - Bleeding Disorders:
Occasionally used in conditions like von Willebrand disease to elevate levels of clotting factors (though not its primary use).
5. Contraindications
- Hyponatremia:
Use can exacerbate low sodium levels. - Fluid Overload Conditions:
Conditions such as congestive heart failure, where additional water retention may be harmful. - Hypersensitivity:
Known allergy to desmopressin or its components.
6. Drug Interactions
- Diuretics:
Concurrent use may alter fluid balance, requiring close monitoring. - Other Agents Affecting Electrolytes:
Medications that alter sodium or water balance may compound the effects of antidiuretic therapy.
7. Side Effects
- Common Side Effects:
- Mild headache, nausea, and abdominal discomfort.
- Nasal irritation (when administered intranasally).
8. Adverse Effects
- Hyponatremia and Water Intoxication:
Overcorrection can lead to dangerously low sodium levels, causing confusion, seizures, or coma if not managed appropriately.
9. Toxicity
- Overdose:
Can result in severe water retention, marked hyponatremia, neurological symptoms, and potentially life-threatening water intoxication. - Management:
Requires immediate intervention with fluid restriction and careful electrolyte monitoring.
10. Role of the Nurse
- Administration:
Ensure proper dosing and correct route of administration (e.g., intranasal vs. injectable forms). - Monitoring:
- Track fluid intake and output meticulously.
- Regularly monitor serum sodium and other electrolyte levels.
- Observe for signs of water intoxication (e.g., headache, confusion, seizures).
- Patient Education:
- Inform patients about the importance of following prescribed fluid restrictions.
- Educate on recognizing early signs of hyponatremia and when to report symptoms.
- Documentation and Communication:
Accurately document all administration details, vital signs, and any adverse reactions, and communicate these findings to the prescribing physician promptly.
Drugs toxic to kidney
Below is an overview of drugs known for their nephrotoxic potential, including the mechanisms by which they can harm the kidney, clinical considerations, and the role of the nurse in preventing and monitoring for kidney injury.
1. Overview of Nephrotoxicity
Nephrotoxicity refers to the damage to kidney tissues resulting in impaired renal function. This can lead to acute kidney injury (AKI) or contribute to chronic kidney disease (CKD). Nephrotoxic drugs may cause harm by:
- Direct tubular injury (e.g., necrosis of renal tubular cells).
- Hemodynamic alterations (e.g., reducing renal blood flow).
- Immune-mediated injury (e.g., interstitial nephritis).
2. Classes of Drugs with Nephrotoxic Potential
Aminoglycosides
- Examples: Gentamicin, Tobramycin, Amikacin
- Mechanism: These antibiotics accumulate in proximal tubular cells, leading to cellular injury and necrosis.
- Clinical Considerations: Monitor serum drug levels and renal function tests.
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
- Examples: Ibuprofen, Naproxen, Diclofenac
- Mechanism: Inhibit prostaglandin synthesis, which can reduce renal blood flow—especially in patients with compromised renal perfusion.
- Clinical Considerations: Avoid in patients with pre-existing kidney disease or volume depletion.
Radiocontrast Agents
- Use: Administered during imaging studies
- Mechanism: Can cause direct tubular toxicity and renal ischemia leading to contrast-induced nephropathy.
- Clinical Considerations: Ensure adequate hydration before and after contrast administration.
Chemotherapeutic Agents
- Example: Cisplatin
- Mechanism: Directly toxic to renal tubular cells, often causing acute tubular necrosis.
- Clinical Considerations: Prehydration protocols and dose adjustments are important to minimize risk.
Antifungal Agents
- Example: Amphotericin B
- Mechanism: Causes renal vasoconstriction and direct tubular damage.
- Clinical Considerations: Use lipid formulations when possible to reduce toxicity and monitor electrolytes.
Calcineurin Inhibitors
- Examples: Cyclosporine, Tacrolimus
- Mechanism: Induce vasoconstriction of the renal arterioles, leading to decreased renal perfusion and chronic interstitial fibrosis.
- Clinical Considerations: Regular monitoring of drug levels and renal function is essential, particularly in transplant patients.
Vancomycin
- Mechanism: High trough levels of vancomycin can be associated with renal injury, likely through direct tubular toxicity.
- Clinical Considerations: Monitor serum levels and adjust dosing appropriately.
Antiviral Agents
- Examples: Acyclovir, Tenofovir
- Mechanism: These agents can precipitate in renal tubules or cause direct toxicity, especially when given in high doses or to patients with pre-existing renal dysfunction.
- Clinical Considerations: Adequate hydration and dosage adjustments are key.
Lithium
- Use: Primarily used in bipolar disorder
- Mechanism: Can cause chronic interstitial nephritis and impair the kidney’s concentrating ability (leading to nephrogenic diabetes insipidus).
- Clinical Considerations: Regular monitoring of renal function and serum lithium levels is necessary.
3. Role of the Nurse in Preventing and Managing Nephrotoxicity
- Assessment:
- Evaluate baseline renal function (e.g., serum creatinine, blood urea nitrogen, electrolytes).
- Identify patients at high risk (e.g., elderly, pre-existing renal impairment, volume-depleted).
- Administration:
- Ensure proper dosing and timing, especially with drugs known to be nephrotoxic.
- Advocate for prehydration strategies before administering contrast media or nephrotoxic chemotherapy.
- Monitoring:
- Regularly assess urine output and monitor vital signs.
- Observe for early signs of AKI (e.g., oliguria, sudden weight gain, edema).
- Monitor laboratory values including serum creatinine and electrolytes.
- Patient Education:
- Inform patients about the importance of hydration and adherence to prescribed doses.
- Educate patients to recognize symptoms of kidney injury (e.g., decreased urine output, swelling, fatigue) and to report them promptly.
- Documentation and Communication:
- Document all medication administration and patient responses.
- Communicate any significant changes in renal function or adverse effects to the healthcare team immediately.
Urinary antiseptics Composition,

Below is an in‐depth overview of urinary antiseptics, focusing on commonly used agents such as nitrofurantoin and methenamine hippurate. These drugs are used primarily to prevent and treat urinary tract infections (UTIs) by creating an environment in the urinary tract that is hostile to bacterial growth.
1. Composition
- Nitrofurantoin:
- A nitrofuran derivative with a chemical structure that includes a furan ring and a nitro group.
- Methenamine Hippurate:
- Consists of methenamine combined with hippuric acid; in acidic urine, methenamine decomposes to yield formaldehyde, a potent antimicrobial agent.
2. Mechanism of Action (Action)
- Nitrofurantoin:
- Bactericidal Activity: Interferes with bacterial enzyme systems by damaging bacterial DNA, cell wall synthesis, and protein synthesis.
- Urinary Concentration: Concentrates in the urine, making it effective against pathogens causing lower UTIs (cystitis).
- Methenamine Hippurate:
- Conversion to Formaldehyde: In an acidic urinary environment, it slowly decomposes to release formaldehyde, which exerts a bactericidal effect by denaturing bacterial proteins and nucleic acids.
- Antiseptic Environment: Helps to reduce bacterial colonization in the urinary tract.
3. Dosage and Route
- Nitrofurantoin:
- Dosage: Typical adult dosing is 50–100 mg every 6 hours (or a modified regimen for extended-release formulations).
- Route: Administered orally.
- Methenamine Hippurate:
- Dosage: Commonly prescribed at about 1 gram orally twice daily, often given as a prophylactic agent for recurrent UTIs.
- Route: Administered orally.
4. Indications
- Nitrofurantoin:
- Primarily indicated for the treatment and prevention of uncomplicated lower urinary tract infections (acute cystitis).
- Often used for prophylaxis in patients with recurrent UTIs.
- Methenamine Hippurate:
- Indicated for prophylactic management of recurrent UTIs, particularly in patients prone to chronic or recurrent cystitis.
- Can be used in cases where traditional antibiotic therapy is either not tolerated or when resistance is a concern.
5. Contraindications
- Nitrofurantoin:
- Contraindicated in patients with significant renal impairment (e.g., creatinine clearance <60 mL/min) due to reduced urinary drug concentration and increased risk of toxicity.
- Not recommended near term in pregnancy due to the risk of hemolytic anemia in the neonate.
- Contraindicated in patients with a known hypersensitivity to nitrofurantoin or related compounds.
- Methenamine Hippurate:
- Contraindicated if the urine is not sufficiently acidic (which is required for conversion to formaldehyde).
- Should be used with caution or avoided in patients with severe renal impairment or those prone to urinary retention.
- Hypersensitivity to methenamine is also a contraindication.
6. Drug Interactions
- Nitrofurantoin:
- May interact with antacids or agents that alter gastric pH, potentially affecting absorption.
- Use with caution in patients with G6PD deficiency, as oxidative stress from the drug could precipitate hemolysis.
- Methenamine Hippurate:
- Interactions can occur with drugs that alkalinize the urine (e.g., sodium bicarbonate), which may reduce its conversion to formaldehyde and thus its efficacy.
- Concomitant use with other urinary antiseptics or antibiotics should be monitored to avoid overlapping toxicity.
7. Side Effects
- Nitrofurantoin:
- Common: Gastrointestinal upset (nausea, vomiting, diarrhea), headache, and dizziness.
- Dermatologic: Rash and pruritus.
- Methenamine Hippurate:
- Common: Gastrointestinal discomfort such as nausea and abdominal cramps.
- May occasionally cause mild headache or rash.
8. Adverse Effects and Toxicity
- Nitrofurantoin:
- Adverse Effects: Rare but serious reactions include pulmonary toxicity (acute or chronic interstitial lung disease), hepatotoxicity, and peripheral neuropathy.
- Toxicity: Overdose can lead to severe pulmonary reactions, liver damage, and neurological effects.
- Methenamine Hippurate:
- Adverse Effects: Excessive formaldehyde production may cause irritation of the bladder and urinary tract, leading to dysuria or hematuria.
- Toxicity: Overdose is uncommon but may lead to systemic formaldehyde exposure, which is potentially harmful.
9. Role of the Nurse
- Administration:
- Verify the correct dosage, formulation, and timing. Ensure that nitrofurantoin is given with food to enhance absorption and reduce gastrointestinal upset.
- For methenamine hippurate, confirm that the patient’s urine pH is appropriate (acidic) for effective conversion to formaldehyde.
- Monitoring:
- Renal Function: Regularly assess renal function (serum creatinine, creatinine clearance) since both drugs depend on renal excretion and urine concentration.
- Signs of Toxicity: Monitor for symptoms of pulmonary distress, liver dysfunction, or neurological changes (especially with nitrofurantoin).
- Urinary Symptoms: Observe for any worsening of urinary symptoms or new onset of dysuria, hematuria, or bladder irritation.
- Patient Education:
- Inform patients about the purpose of the medication and the importance of adhering to the prescribed regimen.
- Advise on maintaining adequate hydration to assist with urinary drug excretion.
- Instruct patients to report any signs of an allergic reaction (rash, difficulty breathing) or any unexpected symptoms such as cough, breathlessness, or unusual fatigue.
- Documentation and Communication:
- Document all assessments, drug administration details, and patient responses.
- Communicate promptly with the healthcare provider if signs of adverse effects or toxicity emerge.
Treatment of UTI – acidifiers and alkalinizers
Below is an overview of using urinary pH modifiers—acidifiers and alkalinizers—as adjunctive measures in the management of urinary tract infections (UTIs). While these agents are not primary antimicrobial therapies, they may help create an unfavorable environment for bacterial growth or alleviate urinary discomfort. Each category is summarized in terms of composition, mechanism of action, dosing, indications, contraindications, potential drug interactions, side effects/adverse effects, toxicity, and the nurse’s role.
Urinary Acidifiers

1. Composition
- Common Agents:
- Ascorbic Acid (Vitamin C): Often used in tablet or powder form.
- Ammonium Chloride: Occasionally used to acidify the urine.
- Note: Some urinary antiseptics (e.g., methenamine hippurate) rely on an acidic environment for activation.
2. Mechanism of Action (Action)
- Lowering Urinary pH:
- Acidifiers work by decreasing the urine pH, which can inhibit the growth of certain uropathogens that prefer a neutral to alkaline environment.
- Enhanced antibacterial activity may also facilitate the activity of some antiseptic agents.
3. Dosage and Route
- Ascorbic Acid:
- Dosage: Typically ranges from 500 to 1000 mg daily (divided doses), depending on clinical need.
- Route: Oral.
- Ammonium Chloride:
- Dosage: Varies by formulation and clinical protocol.
- Route: Oral.
4. Indications
- Adjunct in UTI Management:
- Used to help acidify the urine in patients with recurrent UTIs.
- May enhance the effectiveness of acid-dependent urinary antiseptics.
5. Contraindications
- Patients with a History of Oxalate Stones:
- Excessive acidification may predispose susceptible individuals to stone formation.
- Certain Metabolic Disorders:
- Caution in patients with acid-base balance issues or severe renal impairment.
6. Drug Interactions
- Absorption Interference:
- High doses of ascorbic acid may affect the absorption or efficacy of some medications, though interactions are generally minimal.
7. Side Effects
- Gastrointestinal Disturbances:
- Nausea, abdominal cramps, or diarrhea may occur, especially at higher doses.
8. Adverse Effects and Toxicity
- High-Dose Toxicity:
- Overuse of ascorbic acid can increase the risk of kidney stones in predisposed patients.
- Excess acid load from agents like ammonium chloride may disturb systemic acid-base balance if not monitored.
9. Role of the Nurse
- Assessment and Monitoring:
- Monitor urine pH periodically to assess the effectiveness of acidification.
- Assess for gastrointestinal upset and signs of kidney stone formation.
- Patient Education:
- Explain the rationale behind acidifying the urine and proper dosing.
- Instruct patients to maintain adequate fluid intake.
- Documentation and Communication:
- Record the patient’s urine pH, any side effects, and patient compliance.
- Report adverse events or significant changes in patient status to the healthcare provider.
Urinary Alkalinizers

1. Composition
- Common Agents:
- Sodium Bicarbonate: Available in tablet or liquid form.
- Potassium Citrate: Often used to adjust urinary pH upward.
2. Mechanism of Action (Action)
- Raising Urinary pH:
- Alkalinizers increase the urine pH by providing bicarbonate or citrate, which neutralizes excess acid.
- This may help relieve urinary irritation in patients with painful, highly acidic urine.
- Additionally, in some cases, alkalinization can help prevent the formation of uric acid stones, though its role in UTI management is more symptomatic.
3. Dosage and Route
- Sodium Bicarbonate:
- Dosage: Commonly ranges from 325 mg to 650 mg orally, taken as needed or on a scheduled basis.
- Route: Oral.
- Potassium Citrate:
- Dosage: Typically 10–20 mEq orally, adjusted based on urinary pH targets.
- Route: Oral.
4. Indications
- Symptom Relief:
- Used when acidic urine contributes to bladder irritation or pain.
- Prevention of Stone Formation:
- May be used in patients prone to uric acid stones (an adjunct measure in some UTI cases).
5. Contraindications
- Urease-Producing Infections:
- In UTIs caused by organisms (e.g., Proteus spp.) that already produce alkaline urine, further alkalinization can exacerbate stone formation.
- Metabolic Alkalosis or Hyperkalemia:
- Patients with these conditions should avoid additional alkali load.
- Certain Renal Conditions:
- Caution in patients with compromised renal function.
6. Drug Interactions
- pH-Sensitive Medications:
- Alkalinizers may affect the absorption or efficacy of medications that require a specific pH for optimal absorption (e.g., some antifungal agents).
7. Side Effects
- Gastrointestinal Upset:
- Nausea or bloating may occur.
- Electrolyte Imbalance:
- Particularly with potassium-containing formulations, there is a risk for hyperkalemia if not monitored.
8. Adverse Effects and Toxicity
- Overuse Risks:
- Excessive alkalinization can lead to metabolic alkalosis, causing symptoms such as muscle twitching, confusion, or arrhythmias.
- Sodium load from bicarbonate can contribute to fluid retention and hypertension.
9. Role of the Nurse
- Assessment and Monitoring:
- Regularly monitor urinary pH and electrolyte levels.
- Assess for symptoms of metabolic alkalosis or electrolyte imbalances.
- Patient Education:
- Teach patients the importance of adherence to dosing and potential signs of over-alkalinization.
- Emphasize adequate hydration and proper dietary measures.
- Documentation and Communication:
- Document all findings, including pH levels and any adverse symptoms.
- Communicate promptly with the healthcare provider regarding any significant deviations.