UNIT-2-PBBSC-WATER AND ELECTROLYTE-BIOCHEM.
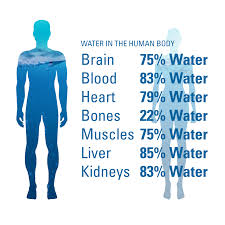
💧 WATER IN THE HUMAN BODY
🌿 Introduction
Water is the most essential nutrient for life — about 60–70% of body weight in adults. Every cell, tissue, and organ needs water to function properly. It is the medium of all biochemical reactions in the body.
🌊 Sources of Water
✅ 1. Dietary Water (Drinking water):
Pure water, beverages like tea, milk, soups, juices, etc.
✅ 2. Food Water:
Fruits (🍉watermelon, 🍊orange), vegetables (🥒cucumber, 🍅tomato), and cooked rice or dal contain a large amount of water.
✅ 3. Metabolic Water:
Produced inside the body during oxidation of nutrients —
• 1 g of fat → 1.07 g of water
• 1 g of carbohydrate → 0.6 g of water
• 1 g of protein → 0.4 g of water
⚗️ Properties of Water
💠 1. Universal solvent: dissolves nutrients, gases, and waste.
💠 2. High heat capacity: maintains body temperature.
💠 3. Neutral pH (≈ 7): safe for metabolic reactions.
💠 4. Transparent and tasteless: helps in visibility and digestion.
💠 5. Cohesive & adhesive nature: aids in blood and lymph flow.
💪 Functions of Water in the Body
💧 1. Transport medium: carries nutrients, hormones, enzymes, and waste through blood and lymph.
💧 2. Temperature regulation: through sweating and evaporation.
💧 3. Lubrication: forms part of synovial fluid (joints), saliva, tears, and mucus.
💧 4. Digestion & absorption: dissolves nutrients and aids in their absorption.
💧 5. Excretion: removes waste via urine, feces, and sweat.
💧 6. Cell structure: maintains cell turgidity and elasticity.
💧 7. Medium for biochemical reactions: supports all metabolic pathways.
⚠️ Water Imbalance Effects
🚱 Dehydration: headache, fatigue, dry skin, low BP, kidney stress.
💦 Over-hydration: electrolyte imbalance → hyponatremia (low Na⁺).
💦 Introduction
Water is the most essential component of life, forming about 60–70% of an adult human body. It plays a vital role in every physiological process — from digestion to temperature regulation. Maintaining the fluid balance (the equilibrium between water intake and water output) is crucial for homeostasis.
🌊 Distribution of Body Fluids
- 🧠 Intracellular Fluid (ICF):
About two-thirds of total body water lies inside the cells. It provides the medium for cellular metabolism and enzyme reactions. - 💉 Extracellular Fluid (ECF):
About one-third of body water is found outside the cells, including:- Interstitial fluid (between cells)
- Plasma (within blood vessels)
- Transcellular fluids (like cerebrospinal, pleural, and synovial fluids)
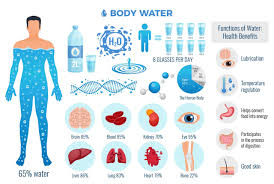
💧 Functions of Water in the Human Body
- 🌡️ Regulates body temperature — through sweating and evaporation.
- 🧫 Solvent for biochemical reactions — allows nutrients, gases, and waste to dissolve.
- 🩸 Maintains blood volume and pressure — keeps circulation and perfusion normal.
- 🧍♀️ Lubricates joints and tissues — synovial and serous fluids protect organs.
- 💨 Aids in digestion and excretion — dissolves nutrients and removes waste through urine, feces, and perspiration.
- ⚡ Transports nutrients and oxygen — via plasma to all body cells.
🥤 Water Intake (Sources of Body Water)
- 🚰 Oral fluids: Drinking water and other beverages.
- 🥗 Food: Fruits, vegetables, soups, etc. contribute to daily intake.
- 🔬 Metabolic water: Formed inside cells as a by-product of metabolism (oxidation of carbohydrates, fats, and proteins).
✅ Average daily intake: ~2500 ml/day (varies by temperature, diet, and activity).
🚽 Water Output (Loss of Body Water)
- 🧻 Urine: Main route of water excretion (~1500 ml/day).
- 💨 Skin: Via sweat and insensible loss (~500 ml/day).
- 🌬️ Lungs: Water vapor lost during breathing (~300 ml/day).
- 🚾 Feces: Around 100–200 ml/day.
⚖️ Balance is achieved when intake = output.
Loss > Intake → Dehydration 💀
Intake > Loss → Overhydration / Edema 💧
⚙️ Regulation of Fluid Balance
- 🧠 Thirst Mechanism (Hypothalamus):
Activated when plasma osmolality rises — signals body to drink water. - 🧬 Antidiuretic Hormone (ADH):
Secreted by posterior pituitary gland — increases kidney water reabsorption to reduce urine output. - 💊 Aldosterone (Adrenal Cortex):
Promotes sodium and water retention, maintaining blood pressure and volume. - 💧 Renin–Angiotensin–Aldosterone System (RAAS):
Activates during low blood pressure — conserves sodium and water. - 🧪 Atrial Natriuretic Peptide (ANP):
Released by heart atria — promotes sodium and water excretion to reduce fluid overload.
⚠️ Imbalances in Water and Electrolytes
- 🧍♀️ Dehydration:
Caused by vomiting, diarrhea, sweating, or inadequate intake.
🔹 Signs: Dry mouth, low BP, tachycardia, confusion. - 💦 Overhydration / Water Intoxication:
Caused by excess water intake or renal failure.
🔹 Signs: Swelling, headache, confusion, hyponatremia. - ⚡ Electrolyte Imbalances:
Sodium, potassium, calcium, and chloride shifts affect muscle and nerve function.
🧠 Clinical Importance for Nurses
- Regular monitoring of intake and output (I/O chart).
- Observation of dehydration or edema signs.
- Maintaining IV fluid therapy as prescribed.
⚡ Electrolytes: Definition, Functions, and Balance in the Human Body
🌟 What Are Electrolytes?
Electrolytes are minerals in the body fluids (blood, urine, tissues) that carry an electric charge (positive or negative). They are vital for life and help maintain many physiological functions.
🧪 When dissolved in water, they form ions — positively charged (cations) and negatively charged (anions) — that regulate critical body processes
.
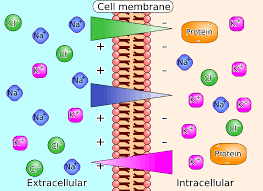
Common electrolytes include:
💧 Sodium (Na⁺)
💧 Potassium (K⁺)
💧 Calcium (Ca²⁺)
💧 Magnesium (Mg²⁺)
💧 Chloride (Cl⁻)
💧 Bicarbonate (HCO₃⁻)
💧 Phosphate (PO₄³⁻)
💪 Main Functions of Electrolytes in the Body
- 🧠 Nerve and Muscle Function:
Electrolytes, especially sodium, potassium, and calcium, transmit electrical impulses that help in nerve conduction and muscle contraction (including the heart). - 💓 Maintaining Fluid Balance:
Sodium and chloride regulate the amount of water inside and outside cells, preventing dehydration or fluid overload. - 🌡️ Maintaining Acid–Base Balance (pH):
Bicarbonate, phosphate, and protein systems help keep blood pH within 7.35–7.45, preventing acidosis or alkalosis. - 💧 Hydration Regulation:
Electrolytes attract water molecules; therefore, their balance ensures optimal cellular hydration and blood volume control. - ⚙️ Metabolic Activities:
Calcium and magnesium serve as cofactors for enzyme reactions, muscle tone maintenance, and blood clotting. - ❤️ Heart Rhythm Stability:
Proper potassium, calcium, and magnesium levels are essential to maintain normal cardiac rhythm and prevent arrhythmias.
⚖️ Electrolyte Balance in the Human Body
The body maintains electrolyte levels through:
🧍♀️ Kidneys, which filter and reabsorb ions according to need.
🩸 Hormones, like aldosterone and antidiuretic hormone (ADH), regulate sodium and water balance.
🥗 Diet, which replenishes electrolytes lost through sweat, urine, and stool.
⚡ Electrolyte Imbalance in the Human Body
Electrolytes are charged minerals that maintain fluid balance, nerve function, and muscle activity. ⚖️
Major electrolytes include Sodium (Na⁺), Potassium (K⁺), Calcium (Ca²⁺), Magnesium (Mg²⁺), Chloride (Cl⁻), Bicarbonate (HCO₃⁻), and Phosphate (PO₄³⁻).
When the level of these ions becomes too high or too low, it leads to electrolyte imbalance, which can cause serious health issues. 🚨
🧠 Causes of Electrolyte Imbalance
- 💧 Dehydration due to vomiting, diarrhea, fever, or excessive sweating
- 💊 Medications such as diuretics, steroids, or laxatives
- ❤️🩹 Chronic diseases — kidney failure, diabetes, liver disorders
- 🍲 Poor dietary intake or malnutrition
- 🧪 Acid–base imbalance in the blood
- ⚕️ Severe burns or trauma causing fluid loss
🧂 Major Electrolytes and Their Imbalances
1. Sodium (Na⁺)
- Normal value: 135–145 mEq/L
- Hyponatremia (↓Na⁺):
- 💧 Causes: Overhydration, renal failure, SIADH
- ⚠️ Symptoms: Confusion, seizures, muscle weakness, nausea, coma
- 💉 Management: Fluid restriction, hypertonic saline if severe
- Hypernatremia (↑Na⁺):
- ☀️ Causes: Dehydration, excessive salt intake, diabetes insipidus
- ⚠️ Symptoms: Thirst, dry mucous membranes, restlessness, seizures
- 💧 Management: Oral or IV hypotonic fluids (e.g., 5% dextrose), monitor Na⁺ closely
2. Potassium (K⁺)
- Normal value: 3.5–5.0 mEq/L
- Hypokalemia (↓K⁺):
- 🍌 Causes: Diuretics, vomiting, diarrhea
- ⚠️ Symptoms: Muscle cramps, weakness, arrhythmias, flattened T wave on ECG
- 💉 Management: Potassium supplements (oral/IV), monitor ECG
- Hyperkalemia (↑K⁺):
- 💊 Causes: Renal failure, ACE inhibitors, tissue breakdown
- ⚠️ Symptoms: Peaked T waves, muscle paralysis, cardiac arrest
- 💊 Management: Calcium gluconate (to protect heart), insulin + glucose, diuretics, dialysis if severe
3. Calcium (Ca²⁺)
- Normal value: 8.5–10.5 mg/dL
- Hypocalcemia (↓Ca²⁺):
- 🥛 Causes: Hypoparathyroidism, vitamin D deficiency, pancreatitis
- ⚠️ Symptoms: Tetany, tingling, muscle spasms, Chvostek’s & Trousseau’s signs
- 💉 Management: IV calcium gluconate, vitamin D supplements
- Hypercalcemia (↑Ca²⁺):
- 🌞 Causes: Hyperparathyroidism, malignancy, excess vitamin D
- ⚠️ Symptoms: Constipation, bone pain, confusion, kidney stones
- 💧 Management: IV normal saline, loop diuretics, corticosteroids, calcitonin
4. Magnesium (Mg²⁺)
- Normal value: 1.5–2.5 mEq/L
- Hypomagnesemia (↓Mg²⁺):
- 🍞 Causes: Alcoholism, malnutrition, diarrhea
- ⚠️ Symptoms: Tremors, seizures, cardiac arrhythmia
- 💉 Management: IV magnesium sulfate, dietary intake of green vegetables, nuts
- Hypermagnesemia (↑Mg²⁺):
- 💊 Causes: Renal failure, excess antacids/laxatives
- ⚠️ Symptoms: Hypotension, muscle weakness, respiratory depression
- 💉 Management: Stop Mg²⁺ intake, give calcium gluconate, dialysis if needed
5. Chloride (Cl⁻)
- Normal value: 98–106 mEq/L
- Hypochloremia:
- 💧 Causes: Vomiting, metabolic alkalosis
- ⚠️ Symptoms: Muscle twitching, slow breathing
- 💉 Management: Replace chloride using saline solution
- Hyperchloremia:
- 💊 Causes: Dehydration, renal disease
- ⚠️ Symptoms: Weakness, deep breathing (Kussmaul respiration)
- 💧 Management: IV fluids, treat underlying cause
6. Phosphate (PO₄³⁻)
- Normal value: 2.5–4.5 mg/dL
- Hypophosphatemia:
- 🍽️ Causes: Malnutrition, alcoholism, DKA treatment
- ⚠️ Symptoms: Muscle weakness, bone pain, confusion
- 💉 Management: Oral or IV phosphate, dietary intake (milk, meat, eggs)
- Hyperphosphatemia:
- 💊 Causes: Renal failure, hypoparathyroidism
- ⚠️ Symptoms: Tetany, calcification of soft tissue
- 💉 Management: Phosphate binders, dialysis, limit phosphate foods
❤️🩹 General Management Principles
- 💦 Maintain fluid balance — adequate hydration or fluid restriction as per condition
- 🩸 Monitor vital signs and ECG for early cardiac changes
- 💉 Regular lab tests — serum electrolytes, renal function
- 🍲 Nutritional support — balanced diet rich in required electrolytes
- ⚕️ Educate patient — avoid self-medication and excessive supplement use
🌿 Prevention Tips
- Drink adequate water 💧
- Eat a balanced diet with fruits, vegetables, and dairy 🍎🥛
- Limit alcohol, caffeine, and processed foods 🚫
- Monitor electrolytes in chronic illness patients 🧾
- Follow medical advice for diuretic or heart medications 💊