🟢B.Sc.Nsg.FIRST YEAR PAPER V-MICROBIOLOGY-OCTOBER 2023 (AUGUST 2023 EXAM SESSION(UPLOAD PAPER NO.3)
MICROBIOLOGY-OCTOBER 2023
⏩I. Elaborate on: (2 x 15 = 30)
🔸1.Write in detail about Hospital Acquired Infection and method of its prevention.
Hospital-Acquired Infection (HAI): Overview
Definition: Hospital-acquired infections (HAIs), also known as nosocomial infections, are infections that patients acquire during the course of receiving healthcare treatment for other conditions. These infections can occur in any healthcare setting, including hospitals, outpatient surgery centers, dialysis facilities, long-term care facilities, and home healthcare.
Common Types of HAIs:
- Catheter-Associated Urinary Tract Infections (CAUTIs)
- Surgical Site Infections (SSIs)
- Central Line-Associated Bloodstream Infections (CLABSIs)
- Ventilator-Associated Pneumonia (VAP)
- Clostridium difficile (C. diff) infections
Causes:
- Pathogens such as bacteria, viruses, fungi, and parasites.
- Contaminated medical equipment and surfaces.
- Transmission via healthcare workers’ hands.
- Invasive procedures and devices (e.g., catheters, ventilators).
Methods of Prevention
Hand Hygiene:
- Regular and thorough hand washing with soap and water.
- Use of alcohol-based hand sanitizers.
- Ensuring hand hygiene compliance among healthcare workers, patients, and visitors.
Use of Personal Protective Equipment (PPE):
- Wearing gloves, gowns, masks, and eye protection when appropriate.
- Proper donning and doffing techniques to prevent contamination.
Sterilization and Disinfection:
- Sterilizing surgical instruments and medical devices.
- Disinfecting surfaces and equipment between patient use.
- Using appropriate disinfectants for different types of pathogens.
Safe Injection Practices:
- Using sterile needles and syringes for each injection.
- Proper disposal of sharps in designated containers.
- Avoiding reuse of single-use vials.
Isolation Precautions:
- Implementing standard precautions for all patients.
- Using contact, droplet, or airborne precautions based on the pathogen.
- Isolating patients with contagious infections.
Antimicrobial Stewardship:
- Judicious use of antibiotics to prevent the development of resistant strains.
- Following guidelines for antibiotic prescribing.
- Monitoring and reviewing antibiotic use.
Proper Care and Maintenance of Medical Devices:
- Ensuring aseptic insertion and maintenance of catheters and central lines.
- Regularly changing and cleaning ventilator circuits.
- Monitoring and caring for surgical sites to prevent infections.
Environmental Hygiene:
- Regular cleaning and disinfection of patient rooms and common areas.
- Using appropriate cleaning agents and methods.
- Ensuring proper ventilation in healthcare facilities.
Education and Training:
- Regular training programs for healthcare workers on infection prevention.
- Patient and family education on hygiene and infection control practices.
- Staying updated on guidelines and best practices.
Surveillance and Reporting:
- Monitoring infection rates and identifying outbreaks.
- Reporting HAIs to relevant health authorities.
- Analyzing data to implement targeted interventions.
Implementation and Monitoring
- Policy Development: Establishing and enforcing infection control policies.
- Auditing and Feedback: Regular audits of compliance with prevention practices and providing feedback.
- Multidisciplinary Approach: Involving infection control teams, healthcare workers, administration, and patients in prevention efforts.
- Continuous Improvement: Using data and feedback to continually improve infection control measures.
By implementing these preventive measures and maintaining vigilance, healthcare facilities can significantly reduce the incidence of HAIs and improve patient outcomes.
🔸2.Draw and explain complete life cycle of Entamoeba histolytica. Write down its pathogencity and laboratory diagnosis.
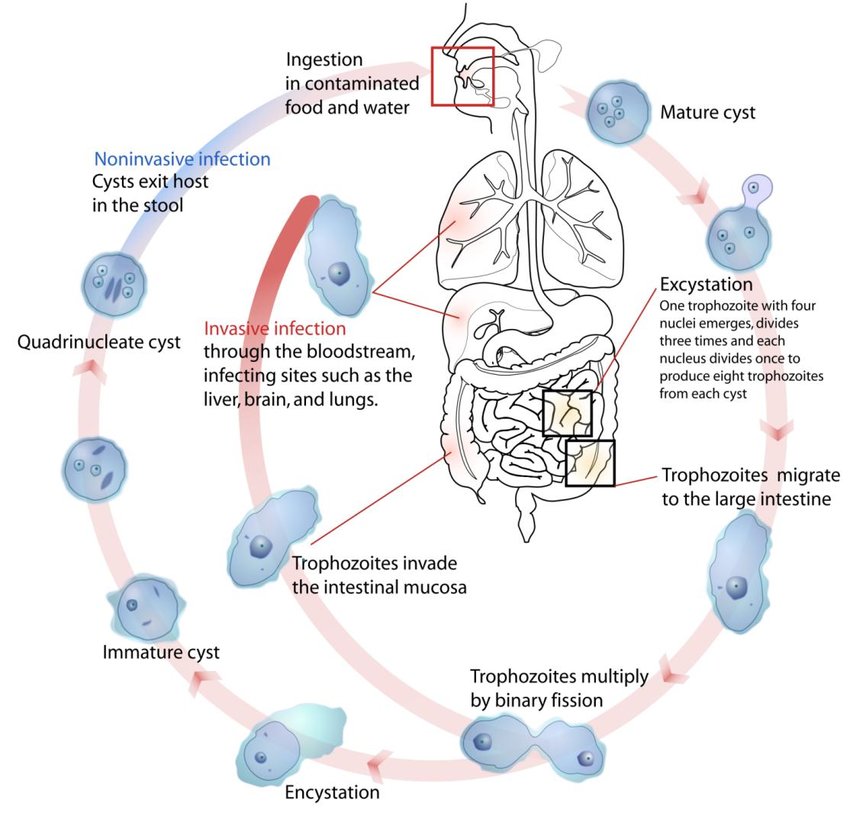
Entamoeba histolytica is the protozoan parasite responsible for amoebiasis, which is a significant cause of morbidity and mortality worldwide. Here’s an overview of its complete life cycle, pathogenicity, and laboratory diagnosis:
Life Cycle of Entamoeba histolytica:
1.Ingestion of Cysts
The infectious stage of Entamoeba histolytica is the cyst, which is resistant to environmental conditions and can survive in water and food. Cysts are typically ingested by humans through contaminated water or food.
2.Excystation in Intestine
Once inside the host’s intestine (usually the large intestine), the cysts excyst and release trophozoites.
3.Trophozoite Stage
Trophozoites are the active, motile form of Entamoeba histolytica. They colonize the mucous layer of the large intestine, where they can feed on bacteria and intestinal contents.
4.Pathogenicity
Some trophozoites can invade the intestinal mucosa, leading to tissue destruction and ulcer formation. This invasive capability is mediated by enzymes that degrade host tissues, allowing the amoeba to penetrate deeper layers of the intestine and potentially enter the bloodstream.
5.Cyst Formation
Trophozoites can revert to the cyst stage under certain conditions (e.g., dehydration, exposure to oxygen) within the intestinal lumen. Cysts are then excreted in feces, completing the life cycle.
Pathogenicity of Entamoeba histolytica:
Intestinal Disease
Entamoeba histolytica causes amoebic colitis, characterized by diarrhea (which may be bloody), abdominal pain, and cramping.
Invasive Disease
Invasive amoebiasis occurs when trophozoites penetrate the intestinal mucosa, leading to amoebic liver abscesses (the most common extraintestinal manifestation), and potentially affecting other organs such as the lungs and brain.
Asymptomatic Carrier State
Some individuals can harbor the parasite without symptoms (asymptomatic carriers) but still shed cysts in their feces, contributing to transmission.
Laboratory Diagnosis:
1.Microscopic Examination of Stool
Direct Examination
Wet mounts or stained smears of fresh stool can reveal cysts (4 nuclei and a characteristic cyst wall) and trophozoites (larger, motile, containing ingested red blood cells).
Concentration Techniques Techniques like sedimentation or flotation can increase the sensitivity of detecting cysts.
2.Serological Tests
Enzyme-linked immunosorbent assay (ELISA) or indirect hemagglutination assays (IHA) can detect antibodies against Entamoeba histolytica in serum, indicating current or past infection. These are useful for diagnosing extraintestinal amoebiasis.
3.Molecular Methods
Polymerase chain reaction (PCR) can detect Entamoeba histolytica DNA in stool samples with high sensitivity and specificity, useful for confirming diagnosis especially in cases where microscopy is inconclusive.
4.Imaging
In cases of suspected amoebic liver abscess, imaging techniques such as ultrasound or CT scan can reveal characteristic lesions in the liver.
Treatment:
Antiprotozoal Drugs
Metronidazole and tinidazole are the drugs of choice for treating invasive amoebiasis and asymptomatic intestinal infection.
Supportive Care
Rehydration and supportive measures may be needed for managing symptoms such as diarrhea and abdominal pain.
⏩II. Write notes on: (5 x 5 = 25)
🔸1.Joseph Lister.
Joseph Lister (1827-1912) was a British surgeon and a pioneer in antiseptic surgery, known for revolutionizing medical practices and significantly reducing surgical mortality rates. Here’s an overview of his contributions and impact:
Contributions and Achievements:
1.Introduction of Antiseptic Techniques
Lister observed that post-operative infections were often caused by airborne bacteria entering wounds. In 1865, he began using carbolic acid (phenol) as an antiseptic during surgery to kill germs and prevent infection.
He developed a technique where instruments, surgical wounds, and dressings were treated with carbolic acid, significantly reducing the incidence of infections.
2.Development of Sterilization Methods
Lister advocated for sterilizing surgical instruments and materials using heat and chemical agents, such as boiling and exposure to steam. This practice helped further minimize the risk of infection.
3.Promotion of Hygiene and Aseptic Practices
Lister emphasized the importance of cleanliness in the operating room and among medical personnel. He promoted washing hands and using clean surgical attire to prevent contamination.
4.Impact on Surgical Outcomes
Lister’s antiseptic methods led to a drastic reduction in surgical mortality and morbidity rates. By eliminating or reducing infections, patients had a higher chance of surviving surgeries and recovering faster.
5.Scientific Legacy
His work laid the foundation for modern antiseptic techniques and infection control in surgery. Lister’s principles influenced the development of sterile techniques, which are standard practices in healthcare today.
Legacy and Recognition:
Baron Lister
In 1883, Joseph Lister was made a Baron by Queen Victoria in recognition of his contributions to medicine and surgery.
Honors and Memorials
Numerous medical institutions and organizations around the world bear his name or have dedicated lectureships and awards in his honor.
Scientific Impact
Lister’s ideas transformed surgical practices, marking a significant shift towards evidence-based medicine and the understanding of microbial causes of infections.
Joseph Lister’s pioneering work in antiseptic surgery not only saved countless lives during his time but also laid the groundwork for modern infection control practices that continue to shape medical care today. His legacy as a visionary surgeon and scientist continues to inspire advancements in healthcare and patient safety globally.
🔸2.Bacterial capsule.
A bacterial capsule is a protective structure surrounding the cell wall of many pathogenic and non-pathogenic bacteria. It is composed of a dense and well-defined layer of polysaccharides (sugar molecules) or glycoproteins that adheres tightly to the bacterial cell surface. Here’s a detailed overview of bacterial capsules:
Structure and Composition:
1.Polysaccharide Matrix
Most capsules are primarily composed of polysaccharides, although some may also contain proteins or glycoproteins. The composition of the capsule can vary greatly between different bacterial species and strains.
2.Physical Properties
Capsules are usually slimy or mucoid in texture, which helps bacteria evade the host immune system by preventing phagocytosis (engulfment and digestion by immune cells).
3.Attachment to Cell Surface
The capsule is firmly attached to the bacterial cell wall and extends outward, surrounding the entire bacterium. It can be visualized under the microscope using special staining techniques.
Functions and Roles:
1.Virulence Factor
Capsules are considered important virulence factors for many pathogenic bacteria. They enhance bacterial pathogenicity by:
Impeding Phagocytosis
Capsules inhibit the ability of phagocytic cells (like macrophages and neutrophils) to engulf and destroy bacteria. This allows bacteria to evade the host immune response and persist in the body.
Resistance to Complement System
Some capsules can resist the complement system, which is part of the innate immune response that helps to destroy pathogens.
2.Protection Against Desiccation
Capsules also provide protection against environmental stresses such as desiccation (drying out), which can be beneficial for bacterial survival outside the host or in harsh conditions.
3.Adhesion and Biofilm Formation
In some bacteria, capsules play a role in adherence to surfaces and in the formation of biofilms. Biofilms are communities of bacteria embedded in a self-produced matrix that adhere to surfaces and can be highly resistant to antibiotics and immune defenses.
Laboratory Detection:
1.Capsule Staining
Capsules can be visualized using specific staining techniques such as:
India Ink Staining
This technique uses a negative stain (India ink) that surrounds the bacteria but does not penetrate the capsule, creating a halo effect around the bacterial cells.
Capsule Staining
Specialized stains like Maneval’s stain or Anthony’s stain can be used to visualize capsules more clearly under the microscope.
2.Biochemical and Molecular Identification
Identification of capsule-specific genes or capsule-associated antigens through biochemical tests or molecular techniques (PCR, sequencing) can also aid in identifying the presence and type of capsule.
Examples of Capsulated Bacteria:
Streptococcus pneumoniae
Known for causing pneumonia and meningitis.
Klebsiella pneumoniae
Often associated with hospital-acquired infections.
Haemophilus influenzae
Causes respiratory tract infections.
Neisseria meningitidis
Causes meningitis and septicemia.
🔸3.Define Incineration with examples.
Incineration is a waste treatment process that involves the combustion of organic substances contained in waste materials. It is commonly used to reduce the volume of waste and to dispose of hazardous or non-hazardous materials in a controlled manner. Here’s a detailed definition along with examples:
Definition and Process:
1.Combustion Process
Incineration involves the combustion of waste materials at high temperatures (typically between 800°C to 1000°C). During combustion, organic materials are converted into ash, gases, and heat.
2.Types of Incinerators
There are different types of incinerators, including:
Solid Waste Incinerators
Used for municipal solid waste (MSW), medical waste, and industrial waste.
Hazardous Waste Incinerators
Specifically designed to handle hazardous materials such as chemicals, pharmaceutical waste, and certain types of industrial waste.
3.Environmental Controls
Modern incinerators are equipped with pollution control devices to minimize emissions of pollutants such as particulates, heavy metals, dioxins, and furans. These controls include scrubbers, filters, and electrostatic precipitators.
Examples of Incineration:
1.Municipal Solid Waste (MSW) Incineration
In many cities around the world, MSW incinerators are used to burn household waste that cannot be recycled or composted.
Example: The EfW (Energy-from-Waste) facility in Edmonton, Canada, incinerates MSW to generate electricity and reduce landfill volume.
2.Medical Waste Incineration
Medical waste, including infectious materials from hospitals, clinics, and laboratories, is often incinerated to prevent the spread of pathogens and to reduce the volume of waste.
Example: Hospitals may have on-site medical waste incinerators or use centralized facilities for safe disposal of medical waste.
3.Industrial Waste Incineration
Industries generate various types of hazardous and non-hazardous wastes that may require incineration for disposal or energy recovery.
Example: Chemical plants may use incinerators to dispose of by-products or waste materials that cannot be reused or recycled.
4.Waste-to-Energy (WtE) Incineration
Some incinerators are designed to generate electricity or heat from the combustion of waste materials, a process known as waste-to-energy.
Example: The Spittelau waste incineration plant in Vienna, Austria, produces district heating and electricity from waste incineration.
Benefits and Considerations:
Volume Reduction
Incineration reduces the volume of waste, particularly useful for densely populated areas with limited landfill space.
Energy Recovery
Waste-to-energy incinerators can generate electricity or heat, contributing to renewable energy production.
Environmental Concerns
Emissions from incinerators, including pollutants and greenhouse gases, require careful management to minimize environmental impact.
Regulatory Compliance
Incineration facilities must adhere to strict regulations regarding emissions and waste handling to protect public health and the environment.
🔸4.Aspergillus fumigatus.
Aspergillus fumigatus is a filamentous fungus belonging to the genus Aspergillus. It is commonly found in the environment, especially in soil, decaying organic matter, and indoor environments where it can grow on damp surfaces. Here’s an overview of Aspergillus fumigatus, including its characteristics, medical significance, and implications:
Characteristics of Aspergillus fumigatus:
1.Morphology
Aspergillus fumigatus has a characteristic filamentous structure with septate hyphae (divided by cross-walls).
It produces conidiophores that bear conidia (asexual spores), which are typically greenish in color and produced in large numbers.
2.Environmental Habitat
It thrives in warm and humid environments, commonly found in soil, compost piles, decaying vegetation, and indoor spaces such as air conditioning systems and HVAC ducts.
3.Pathogenicity
Aspergillus fumigatus is the most clinically significant species among the Aspergillus genus.
It is an opportunistic pathogen, primarily affecting immunocompromised individuals, such as those with weakened immune systems due to HIV/AIDS, chemotherapy, organ transplantation, or underlying lung diseases.
Medical Significance:
1.Infections
Pulmonary Aspergillosis
The most common manifestation is invasive pulmonary aspergillosis (IPA), which can be life-threatening in immunocompromised patients. IPA involves invasion of the lung tissues by Aspergillus hyphae, leading to pneumonia and potentially disseminating to other organs.
Allergic Bronchopulmonary Aspergillosis (ABPA)
This allergic reaction occurs in individuals with asthma or cystic fibrosis, characterized by hypersensitivity to Aspergillus antigens leading to bronchial inflammation and mucus production.
2.Other Infections
Aspergillus fumigatus can also cause infections in other parts of the body, such as sinuses (sinusitis), skin (cutaneous aspergillosis), and invasive infections in wounds or after surgery.
Laboratory Diagnosis:
1.Microscopy
Direct microscopic examination of clinical specimens (e.g., sputum, tissue biopsy) can reveal septate hyphae with characteristic branching patterns.
2.Culture
Aspergillus fumigatus grows readily on fungal culture media at room temperature. Identification is confirmed based on colony morphology, microscopic characteristics, and sometimes molecular methods.
3.Serology and Molecular Tests
Immunological tests (e.g., detection of Aspergillus antigens in serum or respiratory samples) and molecular techniques (PCR assays) can aid in diagnosis, especially in cases where culture may be challenging.
Treatment:
1.Antifungal Therapy
Azoles (e.g., voriconazole)
First-line treatment for invasive aspergillosis.
Amphotericin B
Reserved for severe infections or when azole resistance is suspected.
Echinocandins Used in some cases, particularly for salvage therapy.
2.Surgical Intervention
Invasive infections may require surgical debridement or removal of affected tissues, especially in cases of localized disease or failure of antifungal therapy.
Prevention:
Environmental Control
Reduce exposure to Aspergillus spores by maintaining clean indoor environments, minimizing dust and moisture, and using air filtration systems in hospitals and immunocompromised patient areas.
Prophylactic Antifungal Therapy
Considered in high-risk patients undergoing chemotherapy or stem cell transplantation.
🔸5.VDRL Test.
The VDRL (Venereal Disease Research Laboratory) test is a blood test used to screen for syphilis, a sexually transmitted infection caused by the bacterium Treponema pallidum. Here’s a detailed overview of the VDRL test, its purpose, procedure, interpretation, and considerations:
Purpose of the VDRL Test:
Screening for Syphilis
The VDRL test is primarily used to detect antibodies produced by the body in response to Treponema pallidum, the bacterium that causes syphilis. It is not specific to the bacterium itself but rather detects antibodies against lipoidal material released from damaged host cells and treponemal components.
Procedure:
1.Blood Sample Collection
A blood sample is collected from a vein in the arm using a needle and syringe or via finger prick.
2.Serum Preparation
The blood is centrifuged to separate serum (the clear liquid part of the blood) from cells.
3.Testing Procedure
The serum is tested in the laboratory using the VDRL test kit.
The test involves mixing the patient’s serum with a cardiolipin-cholesterol-lecithin antigen (reagin) and observing for flocculation (clumping) reactions.
Results are typically read visually after incubation and can be semi-quantitative based on the degree of flocculation observed.
Interpretation of Results:
Non-Reactive/Negative
If no flocculation or very minimal flocculation occurs, it suggests the absence of antibodies to syphilis. However, a negative VDRL test does not completely rule out syphilis, especially in early infection or if the immune response is still developing.
Reactive/Positive
Flocculation or clumping indicates the presence of antibodies to syphilis. A positive VDRL test suggests exposure to syphilis at some point in the past or currently active infection. Further confirmatory tests are typically required to differentiate between past and active infections.
Considerations:
Limitations
The VDRL test can yield false-positive results due to factors such as cross-reactivity with other infections (e.g., Lyme disease, malaria, HIV), autoimmune diseases, or certain medications.
Confirmation
Positive VDRL results should be followed up with confirmatory tests, such as the Treponema pallidum particle agglutination assay (TP-PA), fluorescent treponemal antibody absorption test (FTA-ABS), or rapid plasma reagin (RPR) test to distinguish between past and active infections.
Monitoring Treatment
The VDRL test is also used to monitor the effectiveness of treatment for syphilis. A decrease in VDRL titers over time indicates successful treatment response.
⏩III. Short answers on:(10 x 2 = 20)
🔸1.What are the functions of the structure Pili in bacteria
The bacterial structure known as a pilus (plural: pili) serves several important functions in bacteria:
1.Adhesion
Pili play a crucial role in adhesion to host cells or surfaces. They contain adhesin proteins at their tips that bind specifically to receptors on the surface of host cells or to components of the extracellular matrix.
2.Conjugation
Conjugative pili, also known as sex pili, facilitate the transfer of genetic material (plasmids) between bacteria during a process called conjugation. This horizontal gene transfer allows for the spread of antibiotic resistance genes and other beneficial traits among bacterial populations.
3.Motility
Some types of pili, such as type IV pili, are involved in bacterial motility. They extend and retract, enabling twitching motility on surfaces. This movement helps bacteria to colonize new environments and to migrate towards favorable conditions.
4.Biofilm Formation
Pili are essential for the initial attachment of bacteria to surfaces and subsequent formation of biofilms. Biofilms are communities of bacteria embedded in a self-produced extracellular matrix, which protects them from environmental stresses and immune responses.
5.Virulence
Certain pathogenic bacteria use pili to adhere to host tissues and evade the immune system, contributing to the establishment of infection. Pili may also facilitate the colonization of specific niches within the host and the formation of microcolonies.
🔸2.Mention two uses of Inspissation.
Inspissation is a process that involves thickening or concentrating a substance, typically by evaporation or heating. Here are two uses of inspissation:
1.Preparation of Culture Media
Inspissation is used in microbiology to prepare solid culture media. Agar, a common component of culture media, is often inspissated by boiling with water to dissolve it completely and then allowing it to cool and solidify in petri dishes. This process ensures that the agar is evenly distributed and solidified, providing a suitable surface for bacterial or fungal growth.
2.Production of Condensed Milk
In food processing, inspissation is used to produce condensed milk. Milk is heated and evaporated to remove a significant portion of its water content, resulting in a thickened, concentrated milk product with a longer shelf life. This process also enhances the flavor and texture of condensed milk, making it suitable for various culinary uses.
In both cases, inspissation is essential for achieving the desired consistency, concentration, and properties of the final product, whether it’s in microbiological applications or food production.
🔸3.Negative staining.
Negative staining is a technique used in microbiology and electron microscopy to visualize microbial structures, particularly those that are too delicate to withstand the rigors of conventional staining methods. Here’s an overview of negative staining:
Principle:
Negative staining involves staining the background surrounding the specimen rather than staining the specimen itself. The stain used is acidic and negatively charged, such as India ink or nigrosin. These stains do not penetrate cells or structures, creating a contrast between the stained background and the unstained specimen.
Procedure:
1.Preparation of Stain
A drop of acidic stain (India ink or nigrosin) is placed on a clean microscope slide.
2.Mixing with Specimen
A small amount of the microbial sample, usually suspended in water or buffer, is mixed with the stain on the slide. The mixture is gently spread across the slide to create a thin film.
3.Air Drying
The slide is allowed to air dry, which helps in the formation of a thin, uniform film of stained background around the unstained microbial specimen.
4.Visualization
The slide is then examined under a microscope. The unstained microbial cells or structures appear as light or clear against the dark background of the stained area.
Advantages:
Preservation of Structure
Negative staining preserves the natural morphology and structure of microbial cells or delicate structures like capsules, flagella, and bacterial spores because it does not involve the use of harsh chemicals that could distort or damage these features.
Simple and Quick
It is a relatively simple and quick technique compared to other staining methods, requiring minimal preparation and equipment.
Contrast Enhancement
Negative staining provides excellent contrast, making it easier to observe fine details and structures that may be otherwise difficult to visualize using other methods.
Applications:
Capsule Staining
Negative staining is commonly used to visualize bacterial capsules, which are not easily stained by conventional methods due to their composition and structure.
Flagella Staining
It is also useful for visualizing bacterial flagella, which are thin, hair-like appendages used for motility.
Observation of Protozoa and Fungi
Negative staining can be applied to observe various microbial organisms, including protozoa and fungi, providing valuable information about their morphology and characteristics.
Limitations:
Lack of Specificity
Negative staining does not provide specific staining of cellular components like cytoplasmic structures or nuclei, limiting its utility for certain types of microscopic analysis.
Artifacts
Care must be taken to ensure the slide is properly air-dried to avoid artifacts or uneven distribution of the stain, which can affect the clarity of observations.
🔸4.Mention four mode of Transmission of Infection.
Infections can be transmitted through various modes, each playing a crucial role in the spread of pathogens. Here are four common modes of transmission:
1.Direct Contact Transmission
Definition
Direct contact transmission occurs when there is physical contact between an infected individual and a susceptible host.
Examples
This can include touching, kissing, sexual intercourse, and contact with bodily fluids (e.g., blood, saliva, respiratory droplets).
2.Indirect Contact Transmission
Definition
Indirect contact transmission involves the transfer of pathogens from a reservoir to a susceptible host via an intermediary object or surface.
Examples
Common vehicles of transmission include contaminated hands, medical equipment, food, water, and environmental surfaces (fomites).
3.Airborne Transmission
Definition
Airborne transmission occurs when pathogens are carried by small respiratory droplets that remain suspended in the air for long periods or travel distances beyond close contact.
Examples
Respiratory infections like influenza, tuberculosis (TB), and COVID-19 can spread through airborne transmission when infected individuals cough, sneeze, or talk.
4.Vector-Borne Transmission
Definition
Vector-borne transmission involves the transfer of pathogens from one host to another through the bites of vectors such as mosquitoes, ticks, fleas, and flies.
Examples
Diseases like malaria (transmitted by mosquitoes), Lyme disease (transmitted by ticks), and dengue fever (transmitted by mosquitoes) are spread through vector-borne transmission.
These modes of transmission highlight the diverse ways in which infectious agents can spread from one individual to another or from a reservoir to a susceptible host. Understanding these modes is crucial for implementing effective infection prevention and control measures to mitigate the spread of infections in various settings, including healthcare facilities, communities, and beyond.
🔸5.Write about Hanging drop method.
The hanging drop method is a technique used in microbiology and microscopy to observe live, motile microorganisms, particularly bacteria and protozoa, under a microscope. Here’s a brief overview of the hanging drop method:
Principle:
The hanging drop method relies on the principle of creating a small, hanging drop of liquid containing the microorganisms of interest. This drop is suspended from the underside of a coverslip, allowing the microorganisms to move freely and be observed in their natural state without being flattened or immobilized.
Procedure:
1.Preparation of Slide
A clean microscope slide is typically used.
A small amount (usually 1-2 drops) of a suitable liquid medium, such as nutrient broth or physiological saline, is placed in the center of the slide.
2.Preparation of Coverslip
A coverslip is carefully inverted and placed over the drop of liquid on the slide. This creates a chamber with a small air pocket between the slide and the coverslip, which helps to prevent drying out of the drop.
3.Inoculation
A sample containing the microorganisms to be observed is carefully added to the edge of the coverslip where it meets the liquid drop. Capillary action draws the sample into the liquid medium.
4.Observation
The slide is then placed under a microscope, typically using a 40x or 100x objective lens for higher magnification.
The hanging drop allows for observation of the microorganisms in their natural, unstained state, allowing researchers to study characteristics such as motility, shape, and interactions.
Advantages:
Live Observation
Microorganisms are observed in their natural environment without the need for staining or fixation, preserving their motility and physiological characteristics.
High Contrast
The liquid medium surrounding the microorganisms provides high contrast against the background, aiding in visualization under the microscope.
Limitations:
Drying Out
Care must be taken to ensure the hanging drop does not dry out during observation, as this can affect the viability and behavior of the microorganisms.
Single Drop Observation
Each slide typically allows for observation of only one or a few hanging drops at a time, limiting throughput compared to other techniques.
The hanging drop method remains valuable in microbiological research and education for studying live microorganisms and their behaviors, providing insights into motility patterns, cell interactions, and more in a controlled laboratory setting.
🔸6.Define Immunity.
Immunity refers to the ability of an organism to resist or defend against harmful pathogens (such as bacteria, viruses, fungi, and parasites) and their toxins. It is a complex biological defense system that involves various mechanisms and components working together to protect the body from infections and diseases. In brief, immunity can be defined as the state of having sufficient biological defenses to prevent infection, or the ability to mount an effective response when infection occurs, thereby promoting health and survival.
🔸7.Write about Simple Microscope.
DPT stands for Diphtheria, Pertussis (whooping cough), and Tetanus. It refers to a combination vaccine that immunizes against these three bacterial infections. Here’s a brief overview of each component:
1.Diphtheria
Causative Agent
Corynebacterium diphtheriae, a bacterium that produces a toxin affecting the respiratory system.
Transmission
Spread through respiratory droplets from infected individuals.
Symptoms
Causes a thick membrane in the throat, leading to difficulty breathing, heart failure, and nerve damage if untreated.
Vaccine
The diphtheria toxoid vaccine component in DPT stimulates immunity against the diphtheria toxin.
2.Pertussis (Whooping Cough)
Causative Agent
Bordetella pertussis, a bacterium causing severe coughing fits.
Transmission
Spread through respiratory droplets.
Symptoms
Characterized by severe coughing spells, often with a “whooping” sound upon inhalation, vomiting, and exhaustion.
Vaccine
The pertussis component in DPT contains inactivated whole cells of Bordetella pertussis or purified components that induce immunity.
3.Tetanus
Causative Agent
Clostridium tetani, a bacterium found in soil and animal intestines that produces a potent neurotoxin.
Transmission
Enters the body through wounds contaminated with soil, dirt, or feces.
Symptoms
Causes severe muscle stiffness, lockjaw (spasms of the jaw muscles), difficulty swallowing, and potentially fatal respiratory complications.
Vaccine
The tetanus toxoid component in DPT induces immunity against the tetanus toxin.
DPT Vaccine:
Combination Vaccine
DPT is administered as a combination vaccine to protect against diphtheria, pertussis, and tetanus in one injection.
Schedule
It is typically given to infants and young children as part of the routine vaccination schedule. Booster doses may be required in adolescence and adulthood to maintain immunity.
Effectiveness
The DPT vaccine has been highly effective in reducing the incidence and severity of diphtheria, pertussis, and tetanus infections where vaccination coverage is high.
Adverse Reactions:
Mild Reactions
Common side effects include mild fever, redness or swelling at the injection site, and fussiness in infants.
Rare Severe Reactions
Rarely, more serious reactions such as seizures or severe allergic reactions (anaphylaxis) may occur.
🔸8.DPT.
DPT stands for Diphtheria, Pertussis (whooping cough), and Tetanus. It refers to a combination vaccine that immunizes against these three bacterial infections. Here’s a brief overview of each component:
1.Diphtheria
Causative Agent
Corynebacterium diphtheriae, a bacterium that produces a toxin affecting the respiratory system.
Transmission
Spread through respiratory droplets from infected individuals.
Symptoms
Causes a thick membrane in the throat, leading to difficulty breathing, heart failure, and nerve damage if untreated.
Vaccine
The diphtheria toxoid vaccine component in DPT stimulates immunity against the diphtheria toxin.
2.Pertussis (Whooping Cough)
Causative Agent
Bordetella pertussis, a bacterium causing severe coughing fits.
Transmission
Spread through respiratory droplets.
Symptoms
Characterized by severe coughing spells, often with a “whooping” sound upon inhalation, vomiting, and exhaustion.
Vaccine
The pertussis component in DPT contains inactivated whole cells of Bordetella pertussis or purified components that induce immunity.
3.Tetanus
Causative Agent
Clostridium tetani, a bacterium found in soil and animal intestines that produces a potent neurotoxin.
Transmission
Enters the body through wounds contaminated with soil, dirt, or feces.
Symptoms
Causes severe muscle stiffness, lockjaw (spasms of the jaw muscles), difficulty swallowing, and potentially fatal respiratory complications.
Vaccine
The tetanus toxoid component in DPT induces immunity against the tetanus toxin.
DPT Vaccine:
Combination Vaccine
DPT is administered as a combination vaccine to protect against diphtheria, pertussis, and tetanus in one injection.
Schedule
It is typically given to infants and young children as part of the routine vaccination schedule. Booster doses may be required in adolescence and adulthood to maintain immunity.
Effectiveness
The DPT vaccine has been highly effective in reducing the incidence and severity of diphtheria, pertussis, and tetanus infections where vaccination coverage is high.
Adverse Reactions:
Mild Reactions
Common side effects include mild fever, redness or swelling at the injection site, and fussiness in infants.
Rare Severe Reactions
Rarely, more serious reactions such as seizures or severe allergic reactions (anaphylaxis) may occur.
🔸9.Define Hospital Acquired Infection.
A Hospital Acquired Infection (HAI), also known as nosocomial infection, is an infection that develops in a patient during their stay in a healthcare facility or hospital, which was not present or in the incubation period at the time of admission. Here’s a brief definition and overview:
Definition:
Hospital Acquired Infections (HAIs) are infections that occur as a result of healthcare interventions or contact with a healthcare environment, and are not present or in the incubation period at the time of admission. These infections can affect patients in hospitals, long-term care facilities, clinics, and other healthcare settings.
Key Points:
Origin
HAIs can originate from healthcare personnel, other patients, contaminated equipment, or the environment within the healthcare facility.
Types
Common HAIs include infections such as urinary tract infections (UTIs), surgical site infections (SSIs), bloodstream infections, pneumonia, and gastrointestinal infections.
Risk Factors
Factors that increase the risk of HAIs include prolonged hospital stays, invasive procedures (e.g., surgery, urinary catheterization), weakened immune systems, and improper use of antibiotics leading to antibiotic resistance.
Prevention:
Preventing HAIs involves implementing infection control measures such as:
Hand Hygiene
Ensuring healthcare workers and visitors practice proper hand hygiene using soap and water or alcohol-based hand sanitizers.
Environmental Cleaning
Regular cleaning and disinfection of patient rooms, medical equipment, and shared surfaces.
Isolation Precautions
Implementing isolation protocols for patients with known or suspected infections to prevent transmission to others.
Antibiotic Stewardship
Using antibiotics judiciously to prevent the emergence of antibiotic-resistant bacteria.
Education and Training
Providing education to healthcare staff, patients, and visitors about infection prevention practices.
Impact:
HAIs can prolong hospital stays, increase healthcare costs, and contribute to morbidity and mortality in affected patients. Preventing and controlling HAIs is essential for improving patient safety, enhancing healthcare quality, and reducing the burden on healthcare systems.
🔸10.Demonstrate the steps in Hand washing.
Hand washing in is a critical practice to prevent the spread of infections among healthcare workers, patients, and visitors. Here are the steps for effective hand washing in a medical setting:
Steps for Hand Washing in Medicine:
1.Wet Your Hands
Turn on the tap and wet your hands with clean, running water. Adjust the temperature to warm or comfortable.
2.Apply Soap
Apply enough soap to cover all surfaces of your hands. Use antimicrobial soap if available, as it helps to reduce the number of transient microorganisms.
3.Rub Palms Together
Rub your palms together vigorously to create a lather. Ensure the soap covers the front and back of your hands, including between your fingers.
4.Rub Back of Hands
Rub the back of each hand with the opposite palm. Interlace your fingers to clean between them thoroughly.
5.Clean Under Nails
Use your fingertips to scrub under your nails on the palm of the opposite hand. This step is crucial for removing dirt and microorganisms trapped under the nails.
6.Rub Wrists
Rub your wrists with the opposite hand to ensure all surfaces up to the wrists are cleaned.
7.Rub Thumbs
Rub each thumb with the opposite hand in a rotational motion. Ensure both thumbs are thoroughly cleaned.
8.Rub Fingertips
Rub the tips of your fingers in the palm of the opposite hand in a circular motion to clean the fingertips.
9.Rinse Thoroughly
Hold your hands under clean, running water to rinse off all soap and loosened dirt. Start from the fingertips and move towards the wrists, ensuring all soap is removed.
10.Dry Hands
Use a clean, disposable paper towel or hand dryer to dry your hands thoroughly. Pat dry from fingertips to wrists. Use the towel to turn off the faucet if possible to avoid recontaminating your hands.
Duration:
The entire hand washing process should take at least 20-30 seconds, including thorough rinsing and drying. This duration allows for effective removal of dirt, oils, and microorganisms.
When to Wash Hands in Medicine:
Before
Touching a patient, performing aseptic procedures, and handling medications or sterile supplies.
After
Removing gloves, touching patient surroundings (bedrails, door handles), and handling body fluids or contaminated materials.
Importance:
Proper hand washing is crucial in medical settings to prevent healthcare-associated infections (HAIs), protect vulnerable patients, and maintain a safe healthcare environment. It is a fundamental practice that every healthcare worker should adhere to diligently to minimize the spread of infections.