🟢B.Sc. (Nursing) FIRST YEAR PAPER V-MICROBIOLOGY-NOVEMBER 2022 (AUGUST 2022 EXAM SESSION(UPLOAD PAPER NO.4)
MICROBIOLOGY-NOVEMBER-2022
⏩I. Elaborate on: (2 x 15 = 30)
🔸1.Define and classify hypersensitivity. Write in detail about type I hypersensitivity.
Hypersensitivity reactions are exaggerated or inappropriate immune responses to antigens that lead to tissue damage or dysfunction. These reactions are classified into four types based on the immune mechanisms involved:
Type I Hypersensitivity (Immediate or Anaphylactic)
Mechanism:
- Mediated by IgE antibodies.
- Involves mast cells and basophils.
Pathogenesis:
- Sensitization: First exposure to an allergen leads to IgE production by B cells.
- IgE binds to receptors on mast cells and basophils.
- Upon subsequent exposure, the allergen cross-links the bound IgE on mast cells and basophils, causing degranulation and release of histamine and other inflammatory mediators.
Examples:
- Allergic rhinitis (hay fever)
- Asthma
- Anaphylaxis
- Food allergies
Type II Hypersensitivity (Cytotoxic)
Mechanism:
- Mediated by IgG or IgM antibodies.
- Directed against antigens on the surface of cells or extracellular matrix.
Pathogenesis:
- Antibodies bind to cell surface antigens.
- This binding activates complement or recruits effector cells such as macrophages and neutrophils.
- Resulting in cell lysis or tissue damage.
Examples:
- Hemolytic anemia
- Goodpasture’s syndrome
- Graves’ disease
- Myasthenia gravis
Type III Hypersensitivity (Immune Complex-Mediated)
Mechanism:
- Mediated by immune complexes (antigen-antibody complexes).
Pathogenesis:
- Immune complexes are formed in the circulation.
- These complexes deposit in tissues (e.g., blood vessels, kidneys).
- This deposition activates complement and recruits inflammatory cells, leading to tissue damage.
Examples:
- Systemic lupus erythematosus (SLE)
- Rheumatoid arthritis
- Post-streptococcal glomerulonephritis
- Arthus reaction
Type IV Hypersensitivity (Delayed-Type)
Mechanism:
- Mediated by T cells (cell-mediated immunity).
- Does not involve antibodies.
Pathogenesis:
- Sensitized T cells recognize antigens presented by macrophages or dendritic cells.
- This recognition leads to T cell activation and cytokine release.
- The cytokines recruit and activate macrophages and other inflammatory cells, resulting in tissue damage.
Examples:
- Contact dermatitis (e.g., poison ivy)
- Tuberculin skin test (Mantoux test)
- Chronic transplant rejection
- Multiple sclerosis
1.Type I Hypersensitivity (Immediate Hypersensitivity):
Mechanism:
Type I hypersensitivity reactions are mediated by IgE antibodies that are bound to mast cells and basophils. Upon exposure to allergen (antigen), cross-linking of IgE antibodies occurs, leading to release of preformed and newly synthesized mediators.
Allergens:
Common allergens include pollen, dust mites, animal dander, foods (e.g., peanuts, shellfish), and drugs (e.g., penicillin).
Clinical Manifestations:
Type I reactions typically manifest within minutes to hours after exposure and can range from localized symptoms (e.g., urticaria, angioedema) to systemic reactions (e.g., anaphylaxis).
Pathophysiology:
Sensitization:
Initial exposure to an allergen induces B cells to produce IgE antibodies specific to that allergen. These IgE antibodies bind to Fc receptors on mast cells and basophils, sensitizing them.
Activation:
Upon subsequent exposure to the same allergen, it binds to IgE antibodies on sensitized mast cells and basophils, causing cross-linking of IgE antibodies.
Mediator Release:
This cross-linking triggers the release of preformed mediators (histamine, tryptase) stored in granules, as well as synthesis of new mediators (e.g., leukotrienes, prostaglandins) that contribute to inflammation and tissue damage.
Examples:
Localized reactions:
Allergic rhinitis (hay fever), allergic conjunctivitis, urticaria (hives), angioedema (swelling).
Systemic reactions:
Anaphylaxis, which is a severe, life-threatening reaction involving multiple organ systems and rapid onset.
Diagnosis:
Often based on clinical history and supported by specific IgE testing (e.g., skin prick test, serum IgE levels) to identify allergens.
Treatment:
Avoidance: Identifying and avoiding triggers.
Medications: Antihistamines, corticosteroids for acute management; epinephrine (adrenaline) for severe reactions (anaphylaxis).
Immunotherapy: Allergen-specific immunotherapy (desensitization) may be considered for certain allergies.
🔸2.Define sterilization and write in detail about physical methods of sterilization.
Sterilization
refers to the complete destruction or elimination of all forms of microbial life, including bacteria, viruses, fungi, and spores. It ensures that an object or substance is free from viable microorganisms, making it safe for use in medical, laboratory, or industrial settings where contamination could pose significant risks.
Physical methods of sterilization
involve the use of physical agents such as heat, radiation, and filtration to achieve sterilization. These methods are preferred in situations where chemical agents may be harmful or inappropriate. Here are the main physical methods of sterilization:
1.Heat Sterilization:
Moist Heat (Autoclaving):
Autoclaving is the most widely used method of sterilization. It involves subjecting items to high-pressure saturated steam (typically at 121°C) for a specified period (usually 15-20 minutes). This method effectively kills bacteria, fungi, and viruses, as well as spores.
Advantages:
Rapid, reliable, and penetrates well into porous materials.
Disadvantages: Not suitable for heat-sensitive materials.
2.Dry Heat:
Dry heat sterilization involves heating items at temperatures typically ranging from 160°C to 190°C for varying durations. It works by oxidizing cell components and denaturing proteins.
Advantages:
Suitable for heat-stable materials, no moisture residue.
Disadvantages: Longer exposure times compared to moist heat, less effective penetration into materials.
2.Radiation Sterilization:
Ionizing Radiation (Gamma Rays, X-rays):
Ionizing radiation effectively kills microorganisms by causing damage to DNA and other cellular components. It is used for sterilizing heat-sensitive materials, such as medical devices, pharmaceuticals, and some food products.
Advantages:
Penetrates well and sterilizes without heat, suitable for a wide range of materials.
Disadvantages: Requires specialized equipment and handling due to safety considerations.
Ultraviolet (UV) Radiation:
UV radiation is used for surface sterilization in air and water purification systems. It damages DNA and prevents microbial replication, but it has limited penetration and effectiveness against spores.
Advantages: Non-toxic, easy to use for surface sterilization.
Disadvantages: Limited penetration, requires direct exposure to UV light.
- Filtration:
Membrane Filtration:
This method involves passing liquids or gases through filters with pore sizes small enough to trap microorganisms. It is commonly used for sterilizing heat-sensitive liquids (e.g., culture media, pharmaceutical solutions).
Advantages:
Preserves heat-sensitive materials, effective for sterilizing liquids.
Disadvantages: Limited to liquids or gases, pore size selection critical for effectiveness. - Other Physical Methods:
Pasteurization:
Although primarily used for reducing microbial load rather than sterilization, pasteurization involves heating liquids (e.g., milk, beer) to temperatures that kill pathogens without significantly altering taste or quality.
Advantages:
Preserves taste and nutrients of liquids.
Disadvantages: Not suitable for achieving sterilization. High-Pressure Sterilization:
High-pressure processing involves subjecting foods or other materials to extremely high pressures (usually above 100 MPa), which disrupts cellular structures and kills microorganisms.
Advantages: Preserves food quality, effective against bacteria and viruses.
Disadvantages: Limited penetration into solid materials.
Each physical method of sterilization has its specific applications and limitations, depending on the nature of the material to be sterilized and the desired level of microbial reduction or elimination. Proper selection and validation of sterilization methods are crucial to ensure efficacy and safety in various industrial and healthcare settings.
⏩II. Write notes on: (5 x 5 = 25)
🔸1.Acid fast staining.
Acid-fast staining is a laboratory technique used to identify bacteria that belong to the genus Mycobacterium, particularly Mycobacterium tuberculosis and other related species. These bacteria are characterized by their unique cell wall structure, which includes a high content of lipids such as mycolic acids. This lipid-rich cell wall makes them resistant to conventional staining methods and requires a specific staining procedure called acid-fast staining.
Procedure of Acid-Fast Staining:
1.Preparation of Smear:
A sample containing the bacteria (e.g., sputum, tissue biopsy) is spread thinly and evenly onto a clean glass slide. It is then allowed to air dry or may be heat-fixed by passing the slide briefly over a flame.
2.Primary Staining:
The slide is flooded with a primary stain called carbol fuchsin, which is a strong red dye containing phenol. Carbol fuchsin penetrates the lipid-rich cell wall of acid-fast bacteria.
3.Heating:
The slide is gently heated to facilitate the penetration of the stain into the bacterial cells. This heating step is critical for optimal staining and is often done using a steam bath or heating mantle.
4.Cooling and Rinse:
After heating, the slide is allowed to cool to room temperature. Excess stain is then rinsed off with water.
5.Decolorization:
The slide is next treated with an acid-alcohol solution (usually 3-5% HCl in ethanol). This acid-alcohol acts as a decolorizing agent, removing the primary stain from bacteria that do not have the acid-fast cell wall structure.
6.Counterstaining:
Following decolorization, the slide is counterstained with a contrasting color such as methylene blue or brilliant green. This step stains non-acid-fast bacteria and provides contrast to the acid-fast organisms.
7.Microscopic Examination:
Finally, the slide is examined under an oil immersion microscope. Acid-fast bacteria appear bright red or pink against a blue or green background (from the counterstain), while non-acid-fast bacteria appear blue or green.
Significance and Applications:
Diagnosis of Tuberculosis:
Acid-fast staining is particularly important in the rapid diagnosis of tuberculosis (TB). The presence of acid-fast bacilli (AFB) in clinical samples like sputum confirms the presence of Mycobacterium tuberculosis, aiding in the early initiation of treatment.
Detection of Other Mycobacteria:
Besides M. tuberculosis, acid-fast staining is also used to detect other pathogenic mycobacteria such as Mycobacterium leprae (causative agent of leprosy) and Mycobacterium avium complex (MAC).
Veterinary and Environmental Microbiology:
Acid-fast staining is useful in veterinary medicine for diagnosing mycobacterial infections in animals. It is also used in environmental microbiology to identify and study mycobacteria in soil and water samples.
🔸2.Sugar medium.
A sugar medium, in microbiology and biotechnology, refers to a type of growth medium that contains sugars as a primary carbon source for the growth of microorganisms. These media are designed to support the growth of bacteria, fungi, and sometimes other organisms, depending on their nutritional requirements and metabolic capabilities.
Components of a Sugar Medium:
1.Carbon Source (Sugar):
The main component of a sugar medium is one or more sugars that serve as the primary carbon and energy source for microorganisms. Common sugars used include glucose, sucrose, lactose, maltose, and fructose. The choice of sugar(s) depends on the specific microorganism being cultured and its ability to utilize different sugars.
2.Nitrogen Source:
In addition to sugars, nitrogen sources such as peptones, amino acids, or ammonium salts are included in the medium to provide nitrogen for protein synthesis and other metabolic processes.
3.Minerals and Salts:
Sugar media typically contain inorganic salts and minerals essential for the growth and metabolism of microorganisms. These include phosphates, sulfates, magnesium, calcium, potassium, and trace elements like iron, zinc, manganese, and others.
4.pH Buffers:
pH buffers such as phosphates or citrates are often added to maintain a stable pH range suitable for the growth of the microorganisms being cultured.
5.Solidifying Agent (if agar medium):
If the sugar medium is to be used as a solid medium for culturing microbes, agar (or sometimes gelatin) is added as a solidifying agent. Agar provides a semi-solid matrix that allows for the isolation and enumeration of microbial colonies.
Uses and Applications:
Microbial Growth and Identification:
Sugar media are used in laboratories to cultivate microorganisms for various purposes, including isolation, identification, and characterization. Different sugar compositions can help differentiate between microbial species based on their ability to ferment specific sugars.
Industrial Microbiology:
In biotechnology and industrial microbiology, sugar media are employed for the production of various metabolites, enzymes, and other biotechnologically important compounds. For example, yeast fermentation in sugar media is used to produce ethanol, while bacteria can be cultivated for the production of organic acids or enzymes.
Research and Development:
Sugar media play a critical role in research settings for studying microbial physiology, metabolism, and genetics. They are used to investigate microbial growth requirements, metabolic pathways, and the effects of environmental factors on microbial behavior.
Examples of Sugar Media:
Glucose Yeast Extract Agar (GYEA):
Contains glucose and yeast extract, suitable for the growth of a wide range of bacteria and fungi.
Lactose Broth:
Contains lactose as the sole carbon source, used for detecting lactose-fermenting bacteria such as Escherichia coli in water and food samples.
Sabouraud Dextrose Agar (SDA):
Contains dextrose (glucose) and peptones, used for the cultivation of fungi and yeast.
🔸3.CSF- collection procedure.
Collecting cerebrospinal fluid (CSF) is a crucial procedure in diagnosing various neurological conditions and infections. CSF is a clear, colorless fluid that surrounds the brain and spinal cord, providing protection and nourishment. Here’s a detailed outline of the CSF collection procedure:
Indications for CSF Collection:
CSF collection is indicated for:
Diagnosis of meningitis, encephalitis, and other central nervous system infections.
Evaluation of subarachnoid hemorrhage.
Assessment of neurological disorders such as multiple sclerosis.
Measurement of CSF pressure (in cases of suspected intracranial hypertension).
Administration of intrathecal medications or contrast agents.
Equipment Needed:
1.Sterile Collection Kit:
Includes sterile gloves, sterile drapes, antiseptic solution, sterile syringe and needle (typically 20-22 gauge), sterile tubes for CSF collection.
2.Manometer (if measuring CSF pressure):
To measure the opening pressure.
3.Ice Bath:
For immediate placement of CSF tubes to preserve sample integrity (if necessary).
Procedure:
1.Patient Preparation:
Explain the procedure to the patient, ensuring consent is obtained.
Position the patient lying down, usually in a lateral decubitus position (lying on the side).
2.Preparation of Equipment:
Wash hands thoroughly and put on sterile gloves.
Prepare the sterile field and drape the area around the puncture site.
Cleanse the skin over the intended puncture site (usually between L3/L4 or L4/L5 vertebral levels) with an antiseptic solution (e.g., chlorhexidine or iodine).
3.Local Anesthesia:
Administer local anesthesia (usually lidocaine) subcutaneously to numb the skin and deeper tissues at the puncture site.
4.Insertion of Needle:
Insert a sterile needle (usually a spinal needle) between the vertebrae into the subarachnoid space. This is typically done under guidance of anatomical landmarks or with fluoroscopic guidance in some cases.
When the needle enters the subarachnoid space, CSF will begin to flow into the attached syringe.
5.Collection of CSF:
Collect multiple tubes of CSF for various tests (e.g., cell count, protein, glucose, microbiology).
Use gentle suction to collect the fluid. Avoid excessive negative pressure to prevent cell lysis.
If measuring CSF pressure, attach a manometer to the needle and measure the opening pressure with the patient in the same position.
6.Completion of Collection:
Withdraw the needle slowly and apply sterile dressing over the puncture site.
Monitor the patient for any immediate complications (e.g., headache, pain at the puncture site).
7.Handling of CSF Samples:
Label each tube with patient identification, date, and time of collection.
Immediately transport the samples to the laboratory for analysis. If delay is expected, place tubes in an ice bath to preserve sample integrity.
8.Post-procedure Care:
Monitor the patient for several hours for any signs of complications, especially headache or neurological changes.
Provide instructions to the patient regarding post-lumbar puncture care, including fluid intake and activity restrictions.
Complications:
Post-Lumbar Puncture Headache (PLPH):
A common complication due to CSF leakage. It typically resolves spontaneously but can be managed with bed rest, fluids, and analgesics.
Infection:
Risk of introducing pathogens into the CNS. Use sterile technique and prophylactic antibiotics in certain cases.
Hemorrhage:
Rare, but can occur, especially in patients with coagulation disorders or on anticoagulant therapy.
CSF collection is a valuable diagnostic procedure that requires careful technique, adherence to sterile practices, and consideration of potential complications. Properly collected CSF samples provide critical information for diagnosing and managing neurological conditions and infections.
🔸4.Electrophoresis.
Electrophoresis is a laboratory technique used to separate and analyze molecules such as DNA, RNA, proteins, and other charged particles based on their size, shape, and charge. It relies on the principle that charged molecules will migrate through a gel or solution when subjected to an electric field.
Basic Principles:
- Electric Field:
Electrophoresis involves applying an electric field across a medium such as agarose gel (for nucleic acids) or polyacrylamide gel (for proteins). The electric field is created by placing electrodes at opposite ends of the gel.
2.Movement of Charged Molecules:
When the electric field is applied, charged molecules within the sample migrate towards the electrode with opposite charge. For example, negatively charged molecules move towards the positive electrode (anode), while positively charged molecules move towards the negative electrode (cathode).
3.Separation Based on Size and Charge:
The rate of migration through the gel is influenced by the size, shape, and charge of the molecules. Smaller molecules migrate faster and move farther through the gel, whereas larger molecules migrate more slowly and remain closer to the point of origin.
4.Visualization and Analysis:
After electrophoresis, the separated molecules can be visualized using various staining methods specific to the type of molecule (e.g., ethidium bromide for nucleic acids, Coomassie blue for proteins). Bands or spots corresponding to different molecules or fragments are analyzed based on their size and position relative to molecular weight markers or standards.
Types of Electrophoresis:
1.Agarose Gel Electrophoresis:
Used primarily for separating DNA and RNA fragments based on size. Agarose gels have larger pore sizes, suitable for resolving large DNA fragments (e.g., PCR products, genomic DNA).
2.Polyacrylamide Gel Electrophoresis (PAGE):
Used for higher resolution separation of proteins and smaller nucleic acids (e.g., RNA fragments, oligonucleotides). Polyacrylamide gels have smaller pore sizes and can resolve proteins based on their molecular weight.
3.Capillary Electrophoresis:
Utilizes narrow capillary tubes filled with an electrolyte buffer. It is highly automated and allows for rapid separation of DNA, RNA, proteins, and small molecules based on charge and size.
Applications:
DNA Analysis:
Separation of DNA fragments for molecular biology techniques such as restriction mapping, DNA sequencing, and genetic fingerprinting (e.g., PCR products in agarose gel electrophoresis).
Protein Analysis:
Determination of protein size, purity, and quantification (e.g., SDS-PAGE for protein separation).
Clinical Diagnostics:
Detection of genetic mutations (e.g., gel electrophoresis for detecting sickle cell disease mutations) and protein abnormalities (e.g., hemoglobin variants).
Forensic Science:
DNA profiling and analysis of forensic samples.
Research and Development:
Studying gene expression (RNA analysis), protein-protein interactions, and biomarker discovery.
Advantages and Limitations:
Advantages:
High specificity and resolution, versatility across various biomolecules, relatively straightforward technique.
Limitations:
Time-consuming for some applications, requires specialized equipment and expertise, and may be limited by the size of the molecules being analyzed.
🔸5.Cultivation of virus.
The cultivation of viruses involves methods designed to support their replication and propagation in laboratory settings. Viruses are obligate intracellular parasites that require host cells to replicate, as they lack the cellular machinery necessary for metabolism and reproduction. Here’s an overview of the main methods used for cultivating viruses:
1.Cell Culture Techniques:
a. Primary Cell Culture:
Definition:
Primary cells are directly derived from tissues or organs of a host organism. They can be cultured for a limited number of passages before senescence.
Procedure:
Tissue or organ samples are obtained from a suitable host.
Cells are isolated and cultured in appropriate growth media containing nutrients, growth factors, and serum.
Virus inoculum is added to the culture, and cells are monitored for signs of viral infection (e.g., cytopathic effects, CPE).
Applications:
Useful for initial isolation and propagation of viruses from clinical samples or field specimens.
b. Continuous Cell Lines:
Definition:
Continuous cell lines are immortalized cells that can be cultured indefinitely under suitable conditions.
Examples:
HEK293 (Human Embryonic Kidney), Vero (African Green Monkey kidney), MDCK (Madin-Darby Canine Kidney).
Procedure:
Cells are maintained in culture and inoculated with virus.
Viral replication and production are monitored over time.
Applications:
Widely used for routine virus propagation, vaccine production, and basic research.
2.Embryonated Eggs:
Definition:
Fertilized chicken eggs in which the embryo is in the early stages of development.
Procedure:
Virus is inoculated into the allantoic cavity (inside the egg).
The egg is sealed and incubated at a suitable temperature.
After incubation, the allantoic fluid containing the replicated virus is harvested.
Applications:
Commonly used for propagating influenza viruses, Newcastle disease virus, and some strains of adenoviruses.
3.Laboratory Animals:
Definition:
Small laboratory animals, such as mice, rats, guinea pigs, and rabbits, can be used for virus propagation.
Procedure:
Virus is inoculated via various routes (intranasal, intravenous, intraperitoneal) into the animal.
Animals are monitored for signs of infection, and tissues are harvested for virus isolation.
Applications:
Used for studying viral pathogenesis, vaccine development, and testing antiviral therapies.
4.Plant Cell Culture:
Definition:
Certain viruses can infect and replicate in plant tissues and cell cultures.
Procedure:
Plant tissue or cell cultures are inoculated with the virus.
Viral replication is monitored, and symptoms of infection (e.g., chlorosis, necrosis) are observed.
Applications:
Used in agricultural research to study plant viruses and develop disease-resistant crops.
5.Insect Cell Culture (Baculovirus Expression System):
Definition:
Insect cell lines, such as Sf9 (Spodoptera frugiperda), can be infected with baculoviruses.
Procedure:
Insect cells are cultured and infected with recombinant baculovirus carrying the gene of interest.
- Viral replication and protein expression are monitored.
Applications:
Used for recombinant protein production, vaccine development, and basic virology research. - Key Considerations:
Sterility:
All procedures must be conducted under sterile conditions to prevent contamination.
Ethical Considerations:
Animal and human cell culture methods require adherence to ethical guidelines and regulations.
Characterization:
Viruses must be characterized using molecular and serological techniques to confirm identity and purity.
⏩III. Short answers on:(10 x 2 = 20)
🔸1.Anaerobic medium.
An anaerobic medium is a growth medium specifically designed to support the growth of anaerobic microorganisms, which thrive in environments devoid of oxygen or with very low oxygen concentrations. Here’s a brief overview of anaerobic medium:
Composition of Anaerobic Medium:
1.Reducing Agents:
Anaerobic growth media typically contain reducing agents such as thioglycolate, cysteine, or ascorbic acid. These substances help create a low redox potential environment by removing traces of oxygen and promoting anaerobic conditions.
2.Indicator Systems:
To indicate the presence or absence of oxygen, anaerobic media may include oxygen indicators such as resazurin, which changes color in the presence of oxygen.
3.pH Buffers:
pH buffers (e.g., phosphate buffers) maintain a stable pH range optimal for the growth of anaerobic organisms.
4.Nutrients:
Essential nutrients like carbon sources (e.g., glucose, starch), nitrogen sources (e.g., peptones, amino acids), vitamins, and minerals are included to support microbial growth.
5.Solidifying Agent:
If the medium is used as solid medium (agar plates), agar or other gelling agents are added to provide a semi-solid matrix for colony formation.
Types of Anaerobic Media:
1.Fluid Thioglycolate Medium:
Thioglycolate broth supplemented with reducing agents. It supports the growth of a wide range of anaerobic bacteria and can be used to determine oxygen requirements (aerobic, anaerobic, facultative anaerobic) based on growth patterns.
2.Anaerobic Agar Plates:
Agar plates prepared with anaerobic conditions by using an anaerobic jar or chamber, where oxygen is replaced with a gas mixture of hydrogen and carbon dioxide. These plates are used for isolating and enumerating anaerobic bacteria.
3.Gaspak Systems:
These systems create anaerobic conditions in sealed containers by generating hydrogen and carbon dioxide gas mixtures in the presence of a catalyst. They are commonly used in laboratories to maintain anaerobic environments for culturing anaerobic bacteria.
Applications of Anaerobic Medium:
Clinical Microbiology:
Isolation and identification of anaerobic pathogens from clinical specimens, such as abscesses, wounds, and deep tissue infections.
Environmental Microbiology:
Study of anaerobic microorganisms in soil, sediments, and aquatic environments where oxygen availability is limited.
Industrial Microbiology:
Production of anaerobic bacteria for various biotechnological applications, including enzyme production, bioremediation, and fermentation processes.
Research:
Fundamental studies on the physiology, metabolism, and genetics of anaerobic bacteria.
Advantages:
Provides a controlled environment conducive to the growth of anaerobic microorganisms.
Supports the recovery of fastidious anaerobic species that may not grow well in aerobic conditions.
Facilitates the study and characterization of anaerobic pathogens and environmental isolates.
Limitations:
Requires specialized equipment (e.g., anaerobic chambers, gas generators) and techniques to maintain anaerobic conditions.
🔸2.Hide porter’s disease.
Potter syndrome is a rare condition that affects the growth and function of a baby’s kidneys and other internal organs. There are several causes for this condition, but symptoms arise because of too little amniotic fluid in the uterus. This condition is life-threatening for the baby and many infants have a short life expectancy.
Potter syndrome, also known as Potter sequence, is a rare condition that affects how a fetus develops in the uterus. The condition is the result of abnormal kidney growth and function, which affects how much amniotic fluid surrounds the baby during pregnancy. If the absence of kidneys in your baby’s body causes their diagnosis, the condition is fatal. Children who experience mild symptoms or who experience low amniotic fluid during pregnancy (Oligohydroamnios
Tetrad) may survive, but they can develop chronic lung and kidney conditions as they grow.
Potter syndrome can affect any baby since the condition is the result of a lack of amniotic fluid. Some studies found that the condition is more common in male infants.
Potter syndrome affects how your baby’s internal organs develop and function, especially the kidneys. The kidneys are important to remove waste and fluid from your baby’s body. The kidneys create urine (pee), which recycles amniotic fluid in the uterus during fetal development. If your baby’s kidneys aren’t functioning, your baby won’t have enough amniotic fluid surrounding them in your uterus to provide a protective cushion. The lack of amniotic fluid leads to symptoms that affect how their organs and body grow. If your baby’s organs aren’t able to grow completely, they won’t be able to do their job, which leads to life-threatening symptoms.
🔸3.WIDAL test cut off value for O & H antigen.
The interpretation of Widal test results, particularly the cutoff values for the O and H antigens, can vary based on geographical location, local epidemiology, and specific laboratory protocols. However, here are some general guidelines for interpreting Widal test results:
Widal Test Interpretation:
1.O Antigen (Somatic Antigen):
The O antigen of Salmonella indicates current or recent infection. Elevated antibody titers against the O antigen are considered significant for diagnosis.
Common cutoff values for a positive Widal test result for the O antigen include:
A single titer of 1:160 or higher is often considered significant in endemic areas.
In non-endemic areas or for travelers, higher titers (e.g., 1:320 or more) may be used as a cutoff.
2.H Antigen (Flagellar Antigen):
Antibodies against the H antigen of Salmonella typically appear later in the course of infection.
Similar to the O antigen, the cutoff values for the H antigen in the Widal test can vary:
A single titer of 1:160 or higher is commonly considered significant in endemic regions.
-Higher titers (e.g., 1:320 or more) may be used as a cutoff in non-endemic areas or for travelers.
Important Notes:
Baseline Titers:
It’s crucial to know the baseline antibody titers in the local population because some individuals may have naturally elevated titers due to previous exposure or vaccination.
Interpretation:
Widal test results should always be interpreted alongside clinical symptoms, patient history, and other diagnostic tests (such as blood cultures). A rising titer in paired serum samples collected over several days can also be indicative of acute infection.
Geographical Variation:
Cut-off values may differ between regions depending on the prevalence of enteric fever and the circulating strains of Salmonella.
False Positives:
The Widal test can give false-positive results due to cross-reactivity with antibodies against other enteric pathogens or previous vaccination.
Clinical Context :
A single high titer (e.g., 1:160 or higher) for either O or H antigens is suggestive of recent or current infection, but clinical correlation is essential for diagnosis.
Paired serum samples, collected during the acute and convalescent phases, showing a fourfold or greater rise in titer between samples are more specific for diagnosing enteric fever.
🔸4.Tyndallisation.
Tyndallization is a method used for the intermittent sterilization of culture media and other solutions that are heat-sensitive or cannot withstand the high temperatures of autoclaving. This technique was developed by the scientist John Tyndall in the late 19th century and is particularly useful for eliminating heat-resistant spores and certain types of bacteria through a series of boiling and incubation cycles. Here’s a brief overview of the Tyndallization process:
Process of Tyndallization:
1.Boiling:
The medium or solution is initially boiled for a set period (typically 20-30 minutes) to kill vegetative cells and less resistant spores.
2.Incubation:
After boiling, the solution is allowed to stand at room temperature for a period (usually overnight) to promote the germination of any remaining spores into vegetative forms.
3.Re-boiling:
The solution is boiled again on the following day for another 20-30 minutes to kill the newly germinated vegetative cells.
4.Repeat Cycle:
The boiling and incubation cycle is repeated daily for 3-4 days (or more depending on the protocol) to ensure complete elimination of viable microorganisms, including spores that may have survived the initial boiling.
Purpose and Applications:
Heat-sensitive Media:
Tyndallization is suitable for sterilizing heat-sensitive culture media, such as those containing agar or certain nutrient components that would be denatured by autoclaving.
Disadvantages:
It is a time-consuming process compared to autoclaving and requires careful monitoring to ensure effectiveness.
Historical Context:
While largely replaced by more efficient sterilization methods like autoclaving, Tyndallization remains relevant in certain niche applications where autoclaving is not feasible.
🔸5.Name two toxins produced by clostridium tetani.
Clostridium tetani, the bacterium responsible for causing tetanus, produces two main toxins known as tetanolysin and tetanospasmin. Here’s a brief description of each:
1.Tetanospasmin:
Tetanospasmin is the primary neurotoxin produced by Clostridium tetani.
It is a potent toxin that acts on the central nervous system, specifically inhibiting neurotransmitter release at inhibitory synapses.
This inhibition leads to unopposed muscle contraction, causing the characteristic muscle stiffness and spasms seen in tetanus.
Tetanospasmin is responsible for the severe symptoms of tetanus, including lockjaw (trismus), muscle rigidity, and spasms that can lead to respiratory failure if untreated.
2.Tetanolysin:
Tetanolysin, also known as oxygen-labile hemolysin, is another toxin produced by Clostridium tetani.
It is a hemolytic toxin that can cause lysis (breaking open) of red blood cells.
While tetanolysin is less directly involved in the neurological effects of tetanus, it may contribute to tissue damage and the spread of infection in the host.
These toxins play critical roles in the pathogenesis of tetanus, causing the characteristic clinical manifestations associated with the disease. Effective vaccination with tetanus toxoid is crucial in preventing tetanus by inducing protective antibodies against tetanospasmin.
🔸6.Germ tube test.
The germ tube test is a laboratory diagnostic test used primarily to differentiate Candida albicans from other Candida species. Candida albicans is an opportunistic fungal pathogen that can cause infections in humans, particularly in immunocompromised individuals or those with compromised mucosal barriers. Here’s a brief overview of the germ tube test:
Principle of the Germ Tube Test:
Identification of Candida albicans:
Candida albicans is known to produce germ tubes, which are elongated projections or hyphae-like structures that extend from yeast cells when incubated under specific conditions.
Methodology:
The test involves incubating yeast cells (typically obtained from a culture on Sabouraud agar or another appropriate medium) in a specific medium, usually human serum, at 37°C for 1-3 hours.
Observation:
After the incubation period, a drop of the culture is examined microscopically using a light microscope. The presence of germ tubes extending from yeast cells indicates a positive test result.
Interpretation:
Positive Germ Tube Test:
If germ tubes are observed, the isolate is presumptively identified as Candida albicans. Germ tubes are typically long, slender, and arise directly from yeast cells. This test is highly specific for Candida albicans.
Negative Germ Tube Test:
If no germ tubes are observed after the incubation period, the isolate is likely to be another species of Candida (non-albicans Candida species). These species typically do not produce germ tubes under these conditions.
Clinical Significance:
Differentiation:
The ability to differentiate Candida albicans from other Candida species is important clinically because Candida albicans is more commonly associated with invasive infections and is more likely to respond to specific antifungal therapies.
Limitations:
While the germ tube test is specific for Candida albicans, not all strains of Candida albicans may produce germ tubes under the conditions of the test. Therefore, additional confirmatory tests or molecular methods may be required for definitive identification, especially in cases of atypical results or mixed infections.
🔸7.Any four factors affecting growth of bacteria.
Several factors influence the growth of bacteria, affecting their metabolism, reproduction, and overall survival. Here are four key factors:
1.Nutrient Availability:
Bacteria require various nutrients such as carbon, nitrogen, phosphorus, sulfur, vitamins, and minerals for growth.
Availability and composition of these nutrients in the environment significantly impact bacterial growth rates and metabolism.
Limiting factors or excesses can affect growth efficiency and yield.
2.Temperature:
Temperature is a critical factor influencing bacterial growth, as it affects enzyme activity and membrane fluidity.
Bacteria are classified based on their optimal growth temperature: psychrophiles (cold-loving), mesophiles (moderate temperature-loving), and thermophiles (heat-loving).
Extreme temperatures outside the optimal range can slow growth or lead to cell death.
3.pH Level:
Bacteria have specific pH ranges where they grow optimally (pH optima).
Changes in pH can affect enzyme activity, nutrient availability, and membrane integrity.
Acidophiles prefer acidic conditions, while alkaliphiles thrive in alkaline environments. Most bacteria prefer neutral pH (around pH 7).
4.Oxygen Availability (Oxygen Requirement):
Bacteria are classified based on their oxygen requirements: obligate aerobes require oxygen for growth, obligate anaerobes cannot tolerate oxygen, and facultative anaerobes can grow with or without oxygen.
Oxygen availability affects cellular respiration and energy production pathways.
Oxygen toxicity can occur in anaerobic bacteria exposed to oxygen, leading to cell damage or death.
Other factors that can influence bacterial growth include:
Moisture and Water Activity:
Bacteria require water for metabolic processes, and their growth can be inhibited by low water activity or desiccation.
Light:
Some bacteria can utilize light energy (phototrophic bacteria), while others are harmed by exposure to UV light or sunlight.
Environmental Factors:
Presence of toxins, antimicrobial agents, and competition from other microorganisms can also impact bacterial growth.
Understanding these factors is essential in microbiology, as they influence microbial ecology, pathogenesis, and the development of strategies for microbial control and treatment.
🔸8.Vaccine.
A vaccine is a biological preparation that provides immunity to a particular infectious disease. It typically contains a harmless form of the antigen (such as weakened or killed viruses or bacteria) that stimulates the immune system to recognize and remember the pathogen. This preparation allows the immune system to quickly respond if the body is later exposed to the actual disease-causing microorganism, preventing illness or reducing its severity. Vaccines have been instrumental in reducing the incidence of infectious diseases globally and are a cornerstone of public health efforts to protect populations from preventable diseases.
🔸9.Draw the structure of rabies virus.
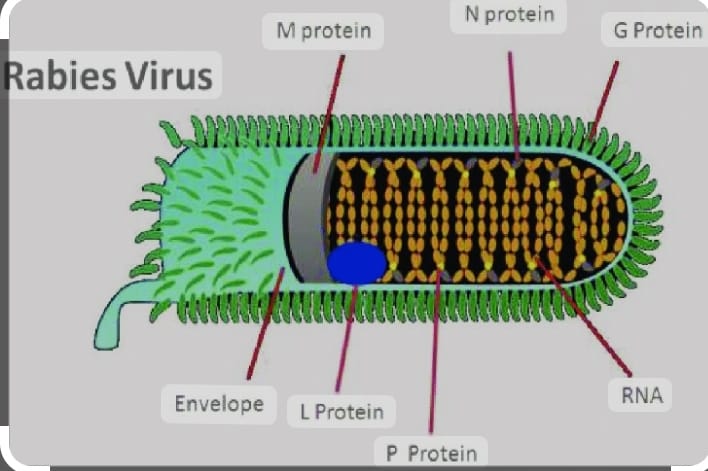
the structure of the rabies .
Description:
RNA Genome:
A single-stranded RNA molecule that carries the genetic information of the virus.
Nucleoprotein (N):
Binds to the viral RNA to form the nucleocapsid core.
Phosphoprotein (P):
Essential for viral replication and assembly.
Matrix protein (M):
Plays a role in maintaining the structural integrity of the virus particle.
Glycoprotein (G):
Forms spikes on the viral surface, facilitating attachment to host cells.
RNA-dependent RNA Polymerase (L):
Enzyme responsible for replicating the viral RNA genome.
Lipid Envelope:
Derived from the host cell membrane, surrounds the nucleocapsid core, and contains glycoprotein spikes.
This representation illustrates the basic components of the rabies virus, emphasizing its RNA genome, protein components, and lipid envelope that collectively contribute to its structure and function as a pathogenic agent.
🔸10.Mention any two method of staining done for fungus.
Staining methods used for fungi in microbiology include:
1.Lactophenol Cotton Blue Stain:
This staining method is commonly used in mycology to visualize fungal structures.
Lactophenol cotton blue is a solution that contains lactophenol (a mounting medium) and cotton blue dye.
It stains fungal structures blue and helps in the identification of fungal morphology, such as hyphae, conidia, and spores.
2.Calcofluor White Stain:
Calcofluor white is a fluorescent dye that binds to cellulose and chitin in fungal cell walls.
It is used in fluorescence microscopy to visualize fungal cell walls, particularly for detecting fungal elements in clinical specimens.
Calcofluor white stain appears bright blue under ultraviolet (UV) light, enhancing the contrast and visibility of fungal structures.
These staining methods are essential in the laboratory for identifying and characterizing fungal species based on their morphological features, aiding in both diagnostic and research applications in mycology.